A placebo-controlled Phase 1/2 trial conducted in East Shanghai has found that administering umbilical cord-derived mesenchymal stem cells reduces frailty in older people.
Stem cells against frailty
These researchers begin by defining frailty as “a state of heightened vulnerability to potential stressors as a consequence of reduction in physiological reserves across multiple systems” [1]. This vulnerability destroys the strength and endurance of older people, exhausting their stamina and greatly increasing their risks of death and disability, and a metric has been determined to measure this [2]. However, while vitamin supplements may help people with nutritional deficiencies, there are no medically approved drugs to treat frailty [3].
As stem cell exhaustion has been pinpointed as a cause of frailty [4], replacement stem cells have been investigated as a possible treatment. In particular, mesenchymal stem cells (MSCs), which are naturally attracted to injury sites [5], appear to be the most promising. MSCs have multiple potential sources for derivation [6], and previous trials have been conducted to treat frailty by using MSCs derived from bone marrow (BM-MSCs), with positive results [7, 8].
This study, however, was conducted on stem cells that were originally derived from the human umbilical cord (HUC-MSCs). These cells are easy to mass produce [9], have been successfully clinically tested against other diseases such as heart failure [10] and arthritis [11], and fight inflammation [12]. This, however, is the first trial of HUC-MSCs for frailty.
Frail adults only
All participants had to meet three criteria: to be between the ages of 60 and 80, to score between 1 and 4 on the Fried frailty scale [2], and to be expected to live another year. A large variety of co-morbidities were screened out, such as uncontrolled diabetes, serious cardiovascular problems, infections, and viral diseases. This was a double-blinded trial from which 80 potential candidates were excluded. 15 patients received placebo, and 15 received MSCs, for 6 months.
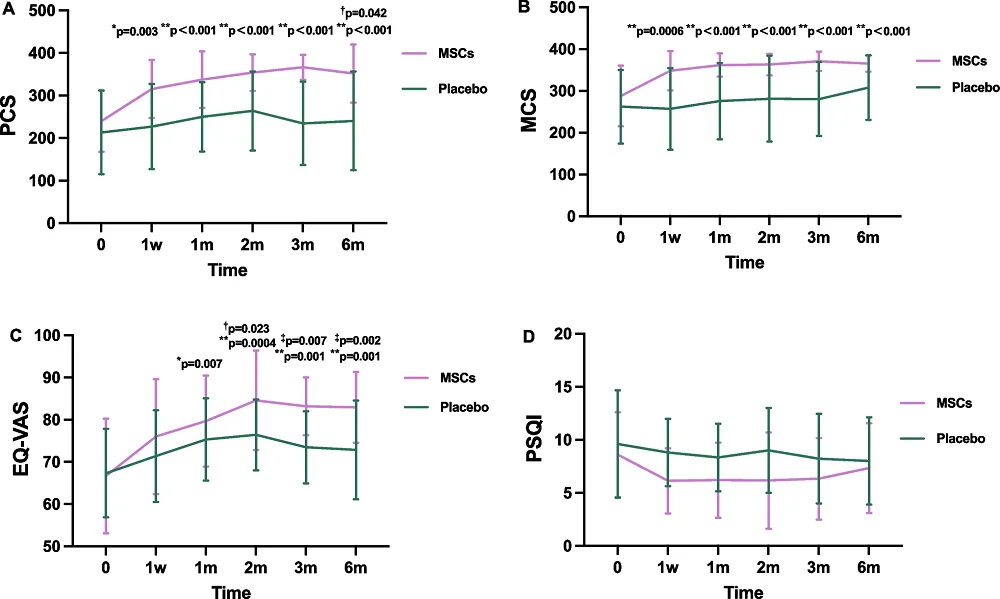
This study measured physical performance by testing grip strength, the timed up-and-go test, walking speed, and the ability to stand up and sit back down. Inflammatory cytokines such as interleukins were also measured, and sleep quality, quality of life, and mental health were also assessed.
Only good significant effects
There were no significant adverse effects. Three participants had suffered from ailments during the trial, two of which were in the placebo group and the third of which had dizziness not related to the MSCs.
Physical function, the primary endpoint of the study, was strongly affected by the MSCs. Even with only 30 total participants, not all of which participated in every assessment, the researchers were able to obtain, against baseline, a p-value of .003 after only one week of treatment and p-values under .001 for 1 and 6 months. Against placebo, the p-value at the end of the 6-month study was .042.
There were possible effects on mental health and sleep quality but those could be statistically attributed to the placebo effect. However, the treatment improved total quality of life with a p-value of 0.002 against placebo at the end of the study.
Cytokines had less clear effects; the placebo group spiked in TNF-α and IL-17 at 6 months while the MSC group did not.
While not all of the endpoints were hit, this study was against frailty, and it is clear from these results that MSCs have beneficial impacts on frailty in human beings. However, this study was conducted in one country among 30 people. Further work, with a larger sample size and more testing sites, will need to be conducted to determine if these results hold up under further scrutiny.
Literature
[1] Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., & Rockwood, K. (2013). Frailty in elderly people. The lancet, 381(9868), 752-762.
[2] Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., … & McBurnie, M. A. (2001). Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56(3), M146-M157.
[3] Dent, E., Morley, J. E., Cruz-Jentoft, A. J., Woodhouse, L., Rodríguez-Mañas, L., Fried, L. P., … & Vellas, B. (2019). Physical frailty: ICFSR international clinical practice guidelines for identification and management. The Journal of nutrition, health and aging, 23(9), 771-787.
[4] Schulman, I. H., Balkan, W., & Hare, J. M. (2018). Mesenchymal stem cell therapy for aging frailty. Frontiers in Nutrition, 5, 108.
[5] Golpanian, S., Wolf, A., Hatzistergos, K. E., & Hare, J. M. (2016). Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiological reviews, 96(3), 1127-1168.
[6] Zhang, J., Huang, X., Wang, H., Liu, X., Zhang, T., Wang, Y., & Hu, D. (2015). The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem cell research & therapy, 6, 1-7.
[7] Golpanian, S., DiFede, D. L., Khan, A., Schulman, I. H., Landin, A. M., Tompkins, B. A., … & Hare, J. M. (2017). Allogeneic human mesenchymal stem cell infusions for aging frailty. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 72(11), 1505-1512.
[8] Tompkins, B. A., DiFede, D. L., Khan, A., Landin, A. M., Schulman, I. H., Pujol, M. V., … & Hare, J. M. (2017). Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double-blind, placebo-controlled clinical trial. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 72(11), 1513-1522.
[9] Sarugaser, R., Lickorish, D., Baksh, D., Hosseini, M. M., & Davies, J. E. (2005). Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem cells, 23(2), 220-229.
[10] Bartolucci, J., Verdugo, F. J., González, P. L., Larrea, R. E., Abarzua, E., Goset, C., … & Khoury, M. (2017). Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circulation research, 121(10), 1192-1204.
[11] Wang, L., Huang, S., Li, S., Li, M., Shi, J., Bai, W., … & Liu, Y. (2019). Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug design, development and therapy, 4331-4340.
[12] Uccelli, A., Pistoia, V., & Moretta, L. (2007). Mesenchymal stem cells: a new strategy for immunosuppression?. Trends in immunology, 28(5), 219-226.





