A review recently published in BMC Biology suggests that taking gerotherapeutics while exercising doesn’t have advantages over separate treatments [1].
Are two approaches better than one?
Exercise is a well-established intervention that ameliorates several aspects of aging. Similarly, other research suggests that certain drugs, known as gerotherapeutics, target biological processes in a way that benefits healthspan and lifespan [2-4].
It is easy to assume that combining these two independent approaches will lead to further health improvements and lifespan extension than each would alone. However, the authors of this recent review took a look through the literature to determine if that is actually the case.
Combining rapamycin and exercise
Rapamycin probably doesn’t need much introduction for people interested in aging and lifespan extension. It inhibits mTOR, a key modulator of cellular and metabolic processes, and this inhibition extends the lifespan of multiple model animals [5].
Rapamycin and its analog, everolimus, are FDA approved for the treatment of kidney transplants and some cancers. However, its prolonged use leads to numerous side effects [6]. Despite mTOR’s involvement in many biological processes, combining exercise with mTOR inhibition by rapamycin treatment has yet to be researched.
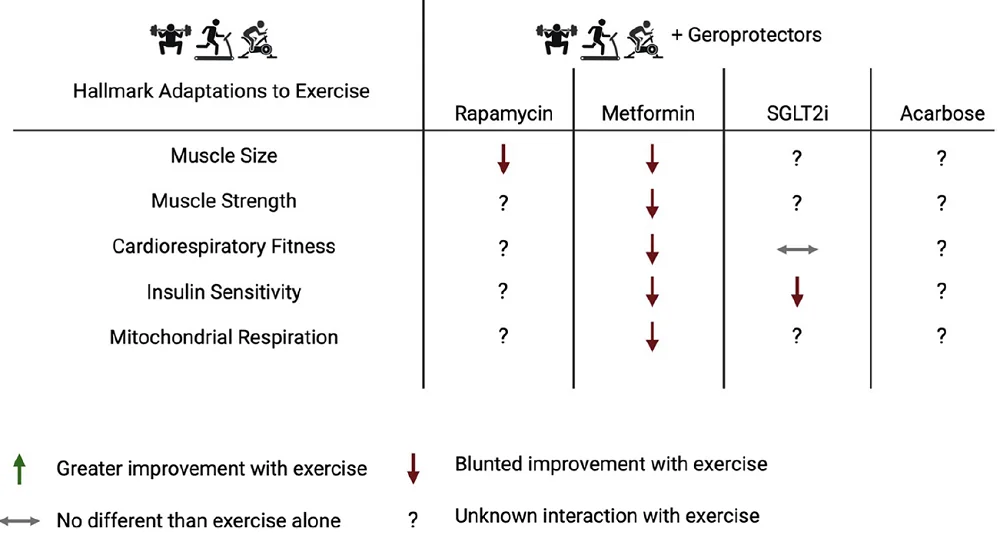
Existing studies suggest that muscle growth might be related to the signaling of mTORC1, one of the complexes in which mTOR exists in the cells [7]. Research both in rodents and humans showed a reduction in “the acute increase in mixed-muscle protein synthesis” when rapamycin was used to inhibit mTORC1. Rodent research also shows reduced muscle growth in rat resistance exercise models [8-10].
Currently, a clinical trial is underway to assess the impact of rapamycin on muscle size and strength in middle-aged to older males, but it is not yet complete.
The researchers summarized that based on current knowledge, combining exercise and long-term rapamycin might not be beneficial to promote muscle mass and glucose tolerance. Conversely, it can lead to negative side effects.
However, they see potential in rapamycin use through overcoming its contradictory effects on different mTOR complexes. In organisms, mTOR exists in two complexes, mTORC1 and mTORC2. Studies on animals suggest that inhibition of mTORC1 is geroprotective. However, at the same time, rapamycin can also inhibit mTORC2, which leads to negative metabolic effects.
The researchers hypothesize that future research might be able to define how to reap the benefits of rapamycin’s impact on mTORC1 while minimizing the side effects it has on mTORC2 inhibition and identify which populations can benefit the most from such interventions.
Other gerotherapeutics
Another gerotherapeutic is metformin, which is commonly given to treat type 2 diabetes. The side effects, while minimal, include vitamin B12 deficiency and gastrointestinal discomfort [11]. Metformin impacts many biological processes, including molecular pathways that are dysregulated in aging, leading to lifespan extension in model organisms [12].
The researchers advise caution when using metformin as a geroprotective drug since the data regarding its impact on humans comes “from preclinical models, patient populations, or those with hyperglycemia, and there is a paucity of data from people with normoglycemia and/or do not have an overt chronic disease” [13]. It is worth waiting for the results of a currently ongoing clinical trial with disease-free participants to determine which populations could potentially benefit from metformin [14].
Currently available data on people without type 2 diabetes suggest that metformin reduces some of the health benefits of exercise: it “inhibits the improvement in cardiovascular risk factors, insulin sensitivity, CRF, and skeletal muscle size, strength, and power” [15-23]. The mechanisms of those effects are not well understood.
The authors note that current studies used metformin in clinically relevant doses. They suggest that testing different doses and regimens might yield different results, possibly more favorable ones.
Alternatively, SGLT2 inhibitors, such as empagliflozin, canagliflozin, and dapagliflozin, can be used as metformin alternatives to lower glucose. While there is a wide amount of data in prediabetic and diabetic populations regarding the effectiveness of SGLT2 inhibitors in glucose regulation and its beneficial effects on the cardiovascular system and weight loss, there is a scarcity of data regarding combining SGLT2 inhibitors and exercise.
The existing data is not very encouraging. One experiment in overweight and obese men and women without diabetes showed that combining dapagliflozin with exercise increased baseline fasting blood glucose and led to reduced improvements in insulin sensitivity following the exercise [24].
The least researched geroprotector and exercise combination involves acarbose, another compound that plays a role in glucose metabolism. The few studies on rodents and diabetic patients show potential in acarbose and exercise combination. However, its effects on healthspan and lifespan in non-patient populations are unknown.
Scarce research, but with potential
The authors note the limited number of studies on the topic. There is a need for more research, especially for females, as most studies use male models. We have addressed this problem in a recent article. They also note that while they focused on skeletal muscles, there is also a need to study the impact of gerotherapeutics and exercise on other tissues.
The authors concluded:
The existing evidence suggests that most leading geroprotective drugs do not cooperate with concurrent exercise training and may limit the healthspan-extending effects of exercise. Opportunities for future research are ripe given few have assessed alternative dosing schemes in the attempt to harness the benefits of exercise and geroprotectors to modulate the biology of aging harmoniously.
Literature
[1] Elliehausen, C. J., Anderson, R. M., Diffee, G. M., Rhoads, T. W., Lamming, D. W., Hornberger, T. A., & Konopka, A. R. (2023). Geroprotector drugs and exercise: friends or foes on healthy longevity? BMC biology, 21(1), 287.
[2] Xu, M., Pirtskhalava, T., Farr, J. N., Weigand, B. M., Palmer, A. K., Weivoda, M. M., Inman, C. L., Ogrodnik, M. B., Hachfeld, C. M., Fraser, D. G., Onken, J. L., Johnson, K. O., Verzosa, G. C., Langhi, L. G. P., Weigl, M., Giorgadze, N., LeBrasseur, N. K., Miller, J. D., Jurk, D., Singh, R. J., … Kirkland, J. L. (2018). Senolytics improve physical function and increase lifespan in old age. Nature medicine, 24(8), 1246–1256.
[3] Miller, R. A., Harrison, D. E., Allison, D. B., Bogue, M., Debarba, L., Diaz, V., Fernandez, E., Galecki, A., Garvey, W. T., Jayarathne, H., Kumar, N., Javors, M. A., Ladiges, W. C., Macchiarini, F., Nelson, J., Reifsnyder, P., Rosenthal, N. A., Sadagurski, M., Salmon, A. B., Smith, D. L., Jr, … Strong, R. (2020). Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI insight, 5(21), e140019.
[4] Harrison, D. E., Strong, R., Alavez, S., Astle, C. M., DiGiovanni, J., Fernandez, E., Flurkey, K., Garratt, M., Gelfond, J. A. L., Javors, M. A., Levi, M., Lithgow, G. J., Macchiarini, F., Nelson, J. F., Sukoff Rizzo, S. J., Slaga, T. J., Stearns, T., Wilkinson, J. E., & Miller, R. A. (2019). Acarbose improves health and lifespan in aging HET3 mice. Aging cell, 18(2), e12898.
[5] Papadopoli, D., Boulay, K., Kazak, L., Pollak, M., Mallette, F., Topisirovic, I., & Hulea, L. (2019). mTOR as a central regulator of lifespan and aging. F1000Research, 8, F1000 Faculty Rev-998. https://doi.org/10.12688/f1000research.17196.1
[6] Johnston, O., Rose, C. L., Webster, A. C., & Gill, J. S. (2008). Sirolimus is associated with new-onset diabetes in kidney transplant recipients. Journal of the American Society of Nephrology : JASN, 19(7), 1411–1418.
[7] Baar, K., & Esser, K. (1999). Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. The American journal of physiology, 276(1), C120–C127.
[8] Drummond, M. J., Fry, C. S., Glynn, E. L., Dreyer, H. C., Dhanani, S., Timmerman, K. L., Volpi, E., & Rasmussen, B. B. (2009). Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. The Journal of physiology, 587(Pt 7), 1535–1546.
[9] Gundermann, D. M., Walker, D. K., Reidy, P. T., Borack, M. S., Dickinson, J. M., Volpi, E., & Rasmussen, B. B. (2014). Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. American journal of physiology. Endocrinology and metabolism, 306(10), E1198–E1204.
[10] Ogasawara, R., Fujita, S., Hornberger, T. A., Kitaoka, Y., Makanae, Y., Nakazato, K., & Naokata, I. (2016). The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Scientific reports, 6, 31142.
[11] Infante, M., Leoni, M., Caprio, M., & Fabbri, A. (2021). Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World journal of diabetes, 12(7), 916–931.
[12] Kulkarni, A. S., Gubbi, S., & Barzilai, N. (2020). Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell metabolism, 32(1), 15–30.
[13] Konopka, A. R., & Miller, B. F. (2019). Taming expectations of metformin as a treatment to extend healthspan. GeroScience, 41(2), 101–108.
[14] Kumari, S., Bubak, M. T., Schoenberg, H. M., Davidyan, A., Elliehausen, C. J., Kuhn, K. G., VanWagoner, T. M., Karaman, R., Scofield, R. H., Miller, B. F., & Konopka, A. R. (2022). Antecedent Metabolic Health and Metformin (ANTHEM) Aging Study: Rationale and Study Design for a Randomized Controlled Trial. The journals of gerontology. Series A, Biological sciences and medical sciences, 77(12), 2373–2377.
[15] Malin, S. K., & Braun, B. (2013). Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme, 38(4), 427–430.
[16] Sharoff, C. G., Hagobian, T. A., Malin, S. K., Chipkin, S. R., Yu, H., Hirshman, M. F., Goodyear, L. J., & Braun, B. (2010). Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. American journal of physiology. Endocrinology and metabolism, 298(4), E815–E823.
[17] Malin, S. K., Gerber, R., Chipkin, S. R., & Braun, B. (2012). Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes care, 35(1), 131–136.
[18] Konopka, A. R., Laurin, J. L., Schoenberg, H. M., Reid, J. J., Castor, W. M., Wolff, C. A., Musci, R. V., Safairad, O. D., Linden, M. A., Biela, L. M., Bailey, S. M., Hamilton, K. L., & Miller, B. F. (2019). Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging cell, 18(1), e12880.
[19] Braun, B., Eze, P., Stephens, B. R., Hagobian, T. A., Sharoff, C. G., Chipkin, S. R., & Goldstein, B. (2008). Impact of metformin on peak aerobic capacity. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme, 33(1), 61–67.
[20] Malin, S. K., Nightingale, J., Choi, S. E., Chipkin, S. R., & Braun, B. (2013). Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity (Silver Spring, Md.), 21(1), 93–100.
[21] Moreno-Cabañas, A., Morales-Palomo, F., Alvarez-Jimenez, L., Ortega, J. F., & Mora-Rodriguez, R. (2022). Effects of chronic metformin treatment on training adaptations in men and women with hyperglycemia: A prospective study. Obesity (Silver Spring, Md.), 30(6), 1219–1230.
[22] Boulé, N. G., Kenny, G. P., Larose, J., Khandwala, F., Kuzik, N., & Sigal, R. J. (2013). Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Diabetologia, 56(11), 2378–2382.
[23] Walton, R. G., Dungan, C. M., Long, D. E., Tuggle, S. C., Kosmac, K., Peck, B. D., Bush, H. M., Villasante Tezanos, A. G., McGwin, G., Windham, S. T., Ovalle, F., Bamman, M. M., Kern, P. A., & Peterson, C. A. (2019). Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging cell, 18(6), e13039.
[24] Newman, A. A., Grimm, N. C., Wilburn, J. R., Schoenberg, H. M., Trikha, S. R. J., Luckasen, G. J., Biela, L. M., Melby, C. L., & Bell, C. (2019). Influence of Sodium Glucose Cotransporter 2 Inhibition on Physiological Adaptation to Endurance Exercise Training. The Journal of clinical endocrinology and metabolism, 104(6), 1953–1966.




