Scientists have identified the proteins FOXF1 and its downstream target FZD4 as potential targets for treating lung cancer. Upregulating them helps to normalize vasculature in the tumor area and suppresses this lethal type of cancer [1].
Lung cancer and vasculature
Lung cancer remains one of the deadliest diseases in the world, accounting for 22% of all cancer deaths. Medicine’s successes against it have been limited, especially against non-small cell lung cancer, a particular type with a five-year survival rate of only 20%. All of this makes developing better treatment strategies imperative.
Lung cancer’s success hinges on the ability of cancer cells to reprogram healthy lung endothelial cells (EC) into tumor-associated endothelial cells (TECs). The latter form leaky, structurally and functionally abnormal blood vessels that promote tumor growth [2]. Cancer cells achieve this by secreting various factors such as vascular endothelial growth factor (VEGF). Normalizing tumor vasculature morphology improves the efficacy of anti-cancer therapies [3]. However, the exact mechanism of how tumor cells change vasculature is not fully understood.
Going down the pathway
In this study, the researchers identified a protein named FOXF1 as decreasing in human and murine endothelial cells associated with non-small cell lung cancer (NSCLC). FOXF1 was expressed by a majority of healthy endothelial cells but only by 5%-10% of tumor-associated ones. Analyzing the Cancer Genome Atlas showed that in a large sample of NSCLC patients, FOXF1 levels were significantly associated with survival.
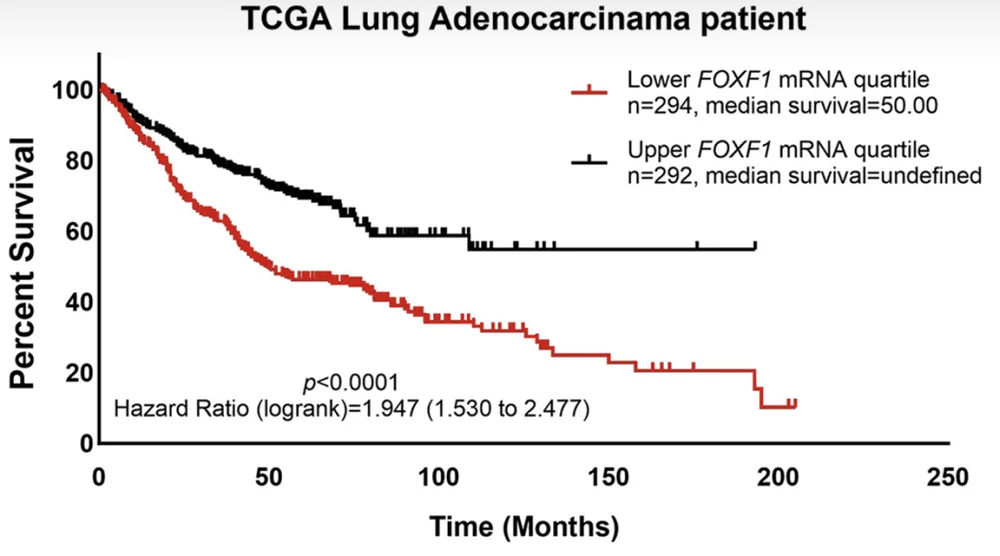
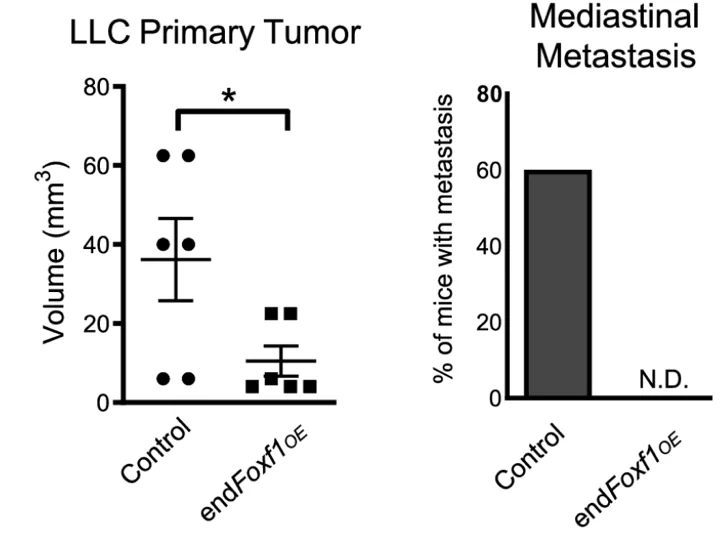
In line with these results, deletion of the Foxf1 gene promoted tumor growth and metastasis in two mouse models of lung cancer and caused cancer-associated structural abnormalities in the lung vasculature.
Overexpression of FOXF1 specific to endothelial cells had the opposite effect. 28 days after mice were inoculated with lung cancer cells, tumors were much smaller in mice with FOXF1 overexpression, and they exhibited virtually no metastases.
The researchers were able to show that FOXF1 does its thing via the canonical Wnt/β-catenin signaling pathway, a mediator of endothelial cells’ proliferation and survival, eventually downregulating another protein, FZD4, by directly binding to the promoter of the gene coding for it. Just like with FOXF1, higher levels of FZD4 in lung cancer patients were associated with better chances of survival.
Nanoparticle administration effective in mice
Finally, the researchers administered FZD4 intravenously, with the help of nanoparticles carrying FZD4-coding DNA plasmids, to FOXF1-deficient mice with lung cancer. The treatment was found to be safe and did not change the histological appearance of endothelial cells in other organs. In lungs, on the other hand, nanoparticles were detected in 70% of cells.
In treated mice, tumor size was decreased to the levels observed in controls with normal FOXF1 expression. The researchers, however, did not investigate the effect of FZD4 administration on mice without a FOXF1 knockout, which would arguably be more clinically relevant. Still, this study is potentially very good news for lung cancer patients.
“We have identified the novel protein FOXF1 that stabilizes blood vessels inside the lung tumors, decreases intertumoral hypoxia and prevents lung cancer metastases,” explained Tanya Kalin, MD, PhD, professor of Child Health and Internal Medicine at the University of Arizona College of Medicine and the senior author of this study. “Since targeting the FOXF1/FZD4 signaling using gene therapy had efficiently decreased lung cancer progression and normalized tumor blood vessels, our next step will be to develop a pharmacological approach to activate this signaling pathway and to move this therapy into clinical trials.”
FOXF1 was highly expressed in normal lung vasculature but was decreased in TEC within non-small cell lung cancers (NSCLC). Low FOXF1 correlated with poor overall survival of NSCLC patients. In mice, endothelial-specific deletion of FOXF1 decreased pericyte coverage, increased vessel permeability and hypoxia, and promoted lung tumor growth and metastasis. Endothelial-specific overexpression of FOXF1 normalized tumor vessels and inhibited the progression of lung cancer. FOXF1 deficiency decreased Wnt/β-catenin signaling in TECs through direct transcriptional activation of Fzd4. Restoring FZD4 expression in FOXF1-deficient TECs through endothelial-specific nanoparticle delivery of Fzd4 cDNA rescued Wnt/β-catenin signaling in TECs, normalized tumor vessels and inhibited the progression of lung cancer. Altogether, FOXF1 increases tumor vessel stability, and inhibits lung cancer progression by stimulating FZD4/Wnt/β-catenin signaling in TECs. Nanoparticle delivery of FZD4 cDNA has promise for future therapies in NSCLC.
Literature
[1] Bian, F., Goda, C., Wang, G., Lan, Y. W., Deng, Z., Gao, W., … & Kalin, T. V. (2024). FOXF1 promotes tumor vessel normalization and prevents lung cancer progression through FZD4. EMBO Molecular Medicine, 1-28.
[2] Dumanskiy, Y. V., Stoliarova, O. Y., Syniachenko, O. V., & Iegudina, E. D. (2015). Endothelial dysfunction of vessels at lung cancer. Experimental oncology, (37,№ 4), 277-280.
[3] Martin, J. D., Seano, G., & Jain, R. K. (2019). Normalizing function of tumor vessels: progress, opportunities, and challenges. Annual review of physiology, 81, 505-534.