A team of researchers has reported in Aging Cell that longer-lived Chinese women have less epigenetic noise in crucial areas of the genome.
Order and disorder

Read More
We have previously reported that the accumulation of epigenetic noise appears to be the main cause of epigenetic alterations. Multiple studies have reported that this noise is correlated with age-related diseases, including Alzheimer’s [1] and vascular disorders [2].
Previous work has studied this entropy in centenarians, although these studies were across the whole genome [3]. The authors of this paper decided to look closer, concluding that these longer-lived people protect certain critical gene areas against noise accumulation.
Less noise, both overall and in crucial places
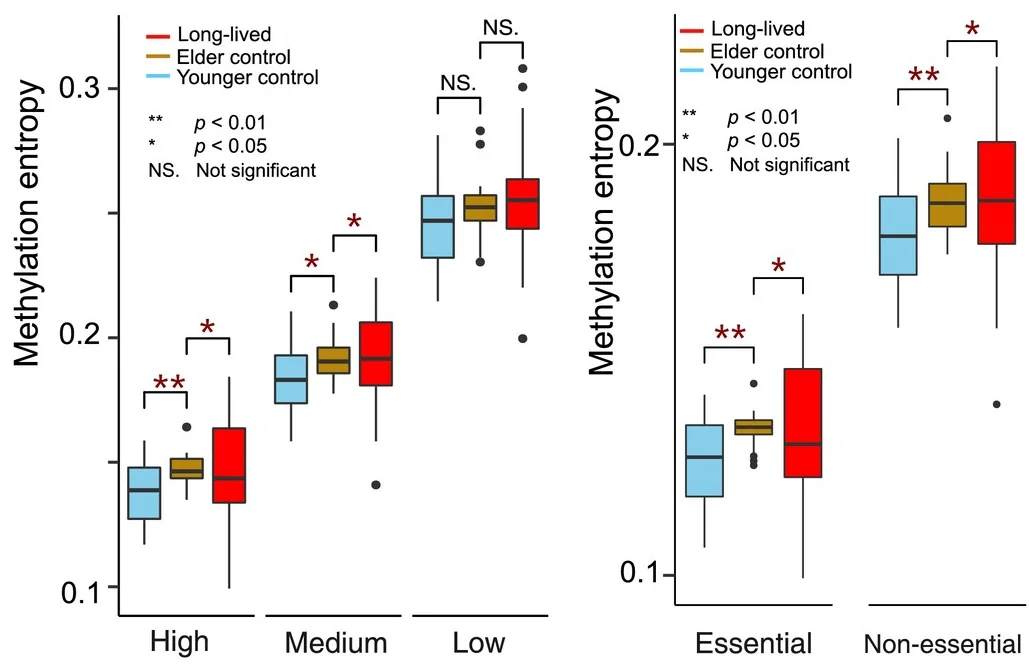
These researchers used samples from a younger group of 34 people aged 45-70, an elder group of 20 people at least 70 years old, and 79 long-lived individuals (LLIs) who were, at the least, nonagenerians: people at least 90 years old. While the overall methylation entropy increased with age in the first two groups, the long-lived individuals had entropy that was generally more like the younger group: people 40 years younger than themselves. The detailed results suggested that this was broadly true across the genes in general.
Among 1842 genes deemed to be essential, the results were even more striking. The LLIs actually had more methylation entropy in nonessential genes than the “elder” group but significantly less entropy among essential genes, although there was a substantial variance within the LLIs.
Further work noted that the better-protected genes are disproportionately gene promoters, which are primarily responsible for RNA transcription. In total, 32.1% of areas were found to have less methylation entropy in LLIs than in the “elder” group. These genes were strongly associated with age-related disorders, including stroke and chronic obstructive pulmonary disorder (COPD). Cancer-related and DNA repair pathways were also represented.
Is there something special about neutrophils?
Neutrophils are the most common kind of blood cell and are responsible for defending against infection. A full 43.1% of the protected regions applied to specific cell types, and neutrophil-related genes were 97.3% of ths group, even though LLIs did not have a significant difference in the number of neutrophils compared to the “elder” group. They did, however, have significantly fewer B cells and CD4+ T cells. The genes that were specific to neutrophils were found to be related to Parkinson’s disease, diabetic cardiomyopathy, and core aspects of proteostasis.
Previous research has suggested that age-related neutrophil changes may make strokes more dangerous [4], and a research team including Steve Horvath has found that neutrophil epigenetic age is particularly connected with Parkinson’s [5]. These researchers hypothesize that the epigenetic protection of these cells in particular is protecting LLIs from age-related diseases, although the details are not yet known.
The researchers note that these results may be only applicable to women. Very long-lived men are considerably rarer than very long-lived women, and they were not available to be part of this cohort. Further studies will need to be conducted to determine if these results are sex-specific.
Literature
[1] Levy, O., Amit, G., Vaknin, D., Snir, T., Efroni, S., Castaldi, P., … & Bashan, A. (2020). Age-related loss of gene-to-gene transcriptional coordination among single cells. Nature metabolism, 2(11), 1305-1315.
[2] Zhang, W., Zhang, S., Yan, P., Ren, J., Song, M., Li, J., … & Qu, J. (2020). A single-cell transcriptomic landscape of primate arterial aging. Nature communications, 11(1), 2202.
[3] Heyn, H., Li, N., Ferreira, H. J., Moran, S., Pisano, D. G., Gomez, A., … & Esteller, M. (2012). Distinct DNA methylomes of newborns and centenarians. Proceedings of the National Academy of Sciences, 109(26), 10522-10527.
[4] Gullotta, G. S., De Feo, D., Friebel, E., Semerano, A., Scotti, G. M., Bergamaschi, A., … & Bacigaluppi, M. (2023). Age-induced alterations of granulopoiesis generate atypical neutrophils that aggravate stroke pathology. Nature Immunology, 24(6), 925-940.
[5] Horvath, S., & Ritz, B. R. (2015). Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging (Albany NY), 7(12), 1130.




