A recent paper published in Nature Aging dives into the gene expression differences between young, middle-aged, and older human ovaries and tests possible interventions to slow down their aging processes [1].
An underexplored area of human aging
Female reproductive aging remains a relatively unexplored area of study. With people living longer and females postponing childbearing, there is a need for research into slowing it down.
The ovaries are indispensable organs in female reproduction, essential in hormone production and fertility. [2] Compared to other organs, ovarian functioning ceases relatively early in life, at around 50 years. However, the decline in its function begins around 20 years earlier, when women reach around the age of 30 [3].
Age-dependent differences in ovaries
In this new study, the researcher focused on exploring human ovaries and the changes to their gene expression (the transcriptome) with age.
One of the biggest challenges to understanding changes in gene expression in human ovarian aging is obtaining tissues for studies. However, these researchers were able to obtain the ovaries of 15 volunteers who donated them following surgery. Those were divided into three groups based on age: young (18-28 years), middle-aged (36-39 years), and older (47-49 years).
Human ovaries are organs that are built from different types of cells [4]. Each cell type has different characteristics and expresses different genes. To understand the aging processes of these different cell types, the authors used techniques that measure gene expression at the single-cell level and compare the gene expression between different age groups and cell types [5].
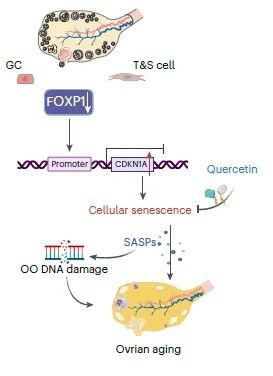
While there were many differences between each cell type, the gene expression analysis generally revealed “significant differences between perimenopausal ovaries and those with reproductive function in young or middle-aged stage.” A more detailed analysis points to cellular senescence as an important player in ovarian aging. The authors also highlight features such as the senescence-associated secretory phenotype (SASP), increased cellular aging hallmarks in aged ovaries, such as SA-β-gal activity and lipofuscin accumulation, and oxidative protein damage.
The SASP molecules secreted by senescent cells are known to lead to inflammation [6]. Those previously observed connections were also observed in this research, as the researchers observed increased levels of inflammation-related molecules. An increase in inflammation and SASP molecules can lead to the spreading of the senescent phenotype and oocyte damage, which was supported by the observation that DNA damage systems were also activated in aged oocytes.
FOXP1, the crucial regulator
After analyzing the common molecular processes and gene expression in different types of ovarian cells, the researchers looked for the transcription factor regulating these processes. They identified “FOXP1 as a crucial TF regulating cellular senescence in the ovary” and observed that aging decreased FOXP1 levels.
They also tested FOXP1 in mice. The depletion of FOXP1 in granulosa cells led to expedited ovarian aging, senescence-associated changes in gene expression patterns, increased levels of senescent markers, and more granulosa cells dying by apoptosis. However, they noted this experiment’s limitations, as knocking down FOXP1 in mice cannot fully replicate human ovarian aging.
Quercetin’s anti-aging effect
The authors went a step further and tested potential anti-aging drugs and their effects on the ovarian reserve in middle-aged mice and human ovarian granulosa tumor cell lines. They tested fisetin, quercetin, and dasatinib, which are already known to have anti-aging properties [7]. First, testing in human cell lines showed that quercetin and fisetin “delayed FOXP1 gene silencing-induced cellular senescence,” improved cell proliferation, and decreased levels of a DNA damage marker. Quercetin also activated FOXP1 expression, suggesting that it can inhibit senescence.
Those promising results prompted researchers to test quercetin in reproductively aged (36-week-old and 48-week-old) mice. These ages correspond roughly to 38-40 and 48-50 in humans. Mice received one month of quercetin treatment without any side effects. Follow-up testing showed improvements in the ovarian reserves of 36-week-old, but not 48-week-old, mice.
Additionally, quercetin-treated 36-week-old mice were able to carry more successful pregnancies compared to the control group. The lack of improvement observed in the 48-week-old mice is probably due to the limited number of follicles, the sacs that contain immature eggs, in those old mice.
Overall, this study contributed to a better understanding of female aging processes. The comprehensive analysis conducted by the authors allowed them to create “single-cell and spatial transcriptomic maps of human ovaries across different age groups.” These maps allowed for examining gene expression changes and differences in eight types of human ovarian cells.
Our study deepens the understanding of human ovarian aging, providing a valuable resource for investigating potential therapeutic interventions. Moving forward, we aim to explore FOXP1 as a potential target for both the diagnosis and treatment of human ovarian aging.
Literature
[1] Wu, M., Tang, W., Chen, Y., Xue, L., Dai, J., Li, Y., Zhu, X., Wu, C., Xiong, J., Zhang, J., Wu, T., Zhou, S., Chen, D., Sun, C., Yu, J., Li, H., Guo, Y., Huang, Y., Zhu, Q., Wei, S., … Wang, S. (2024). Spatiotemporal transcriptomic changes of human ovarian aging and the regulatory role of FOXP1. Nature aging, 10.1038/s43587-024-00607-1. Advance online publication.
[2] Baerwald, A. R., Adams, G. P., & Pierson, R. A. (2012). Ovarian antral folliculogenesis during the human menstrual cycle: a review. Human reproduction update, 18(1), 73–91.
[3] Broekmans, F. J., Knauff, E. A., te Velde, E. R., Macklon, N. S., & Fauser, B. C. (2007). Female reproductive ageing: current knowledge and future trends. Trends in endocrinology and metabolism: TEM, 18(2), 58–65.
[4] Hsueh, A. J., Kawamura, K., Cheng, Y., & Fauser, B. C. (2015). Intraovarian control of early folliculogenesis. Endocrine reviews, 36(1), 1–24.
[5] Tabula Muris Consortium (2020). A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature, 583(7817), 590–595.
[6] Muñoz-Espín, D., & Serrano, M. (2014). Cellular senescence: from physiology to pathology. Nature reviews. Molecular cell biology, 15(7), 482–496.
[7] Di Micco, R., Krizhanovsky, V., Baker, D., & d’Adda di Fagagna, F. (2021). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature reviews. Molecular cell biology, 22(2), 75–95.





