In Nature Communications, researchers have explained how epigenetic drift and disorder are associated with shorter lifespans across species.
When randomness is a problem
We have previously reported on research demonstrating that epigenetic clocks are most likely to be measuring noise. This paper builds on that concept, citing previous research showing that this gradual epigenetic drift is a strong influence on the lifespan of mammals [1] and that it can be broadly used as an epigenetic clock [2].

Read More
Proper DNA methylation, the fundamental biological mechanic behind epigenetics, is part of genomic stability maintenance [3] and is the reason why tissues perform specific functions. The density of methylation sites in certain areas has also been found to be associated with longevity [4], and this relationship may be part of the data used by a recently built tool to predict mammalian lifespan.
This research takes a closer look at this concept, comparing the lifespan of various mammals to the rates at which their epigenomes accumulate drift.
Disorder accumulates in mammals
The researchers began their experiments by first studying the genomes of rats, mice, dogs, and baboons. Interestingly, in samples taken from all four species, some regions became less disordered with time, although considerably more accumulated disorder. As expected, rats and mice, being considerably shorter-lived, were found to accumulate disorder much faster than dogs and baboons, which have similar, longer, lifespans.
However, not all of these species began with the same amount of measured epigenetic disorder. Rats and baboons appeared to share similar amounts at young ages, and mice and dogs shared similar amounts as well, despite their differences in lifespan. Dogs had many considerably denser methylation sites than the other three species, and in dogs and baboons, the relationship between site density and methylation rate was largely flat.
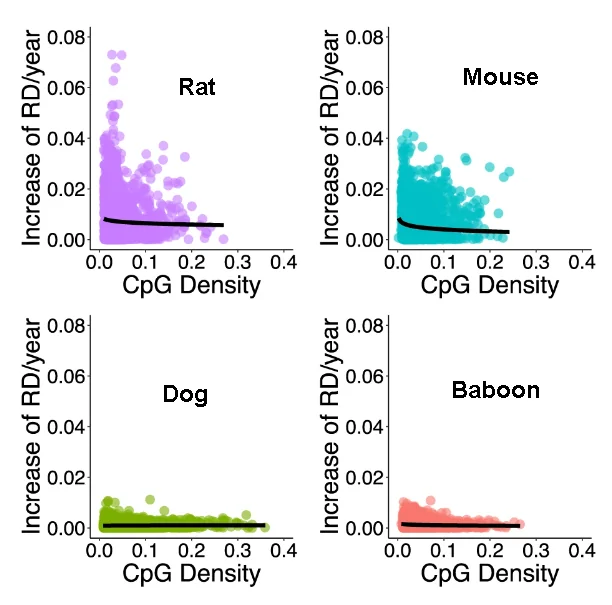
Site density and disorder were also interlinked, particularly in rats and mice. In these short-lived species, disorder accumulated more rapidly in their less-dense sites, and they have fewer dense sites than longer-lived species as well.
In rats, mice, and dogs, the most strongly affected genes were broadly found to be associated with gene transcription regulation and activity along with DNA binding and organismal development. This suggests that this epigenetic noise may be affecting genomic stability over time.
Epigenetic density is not protection
While the density of methylation sites was broadly associated with reduced regional disorder, most strongly in rats and mice, it clearly does not confer substantial protection against accumulating noise: even in their densest regions, rodents accumulated disorder considerably faster than longer-lived species.
Just as longer-lived species have documented protections against genomic instability [5], longer-lived species clearly have epigenetic protections that shorter-lived animals lack. If their mechanisms can be fully identified and explained, it may be possible to transfer them to our pets and, ultimately, to ourselves.
Literature
[1] Mendelsohn, A. R., & Larrick, J. W. (2017). Epigenetic drift is a determinant of mammalian lifespan. Rejuvenation research, 20(5), 430-436.
[2] Mayne, B., Berry, O., Davies, C., Farley, J., & Jarman, S. (2019). A genomic predictor of lifespan in vertebrates. Scientific Reports, 9(1), 1-10.
[3] Bird, A. P. (1986). CpG-rich islands and the function of DNA methylation. Nature, 321(6067), 209-213.
[4] McLain, A. T., & Faulk, C. (2018). The evolution of CpG density and lifespan in conserved primate and mammalian promoters. Aging (Albany NY), 10(4), 561.
[5] Tian, X., Firsanov, D., Zhang, Z., Cheng, Y., Luo, L., Tombline, G., … & Gorbunova, V. (2019). SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell, 177(3), 622-638.