Scientists publishing in Nature have explained a key pathway that leads to immune dysfunction and neurodegeneration in aging.
Feeling the STING
Throughout this paper, the researchers cite other papers demonstrating a cause-and-effect relationship between initial damage and long-term effects. Perturbed mitochondria release their mitochondrial DNA (mtDNA) into the internal liquid (cytosol) of microglia, the helper cells that are responsible for necessary brain functions [1]. This activates the cyclic GMP–AMP synthase (cGAS) found in these cells’ cytosol [2], which leads to the activation of immune-related STING signals. This activation drives inflammation, senescence [3], and ultimately inflammaging, the age-related chronic inflammation that drives many other aspects of aging [4].
To tie these facts together and more thoroughly understand the role of STING, the researchers performed experiments on human cells and on mice.
Directly blocking STING is effective in models
The first experiment discussed in this study used human fibroblasts that had been exposed to strong radiation, which is a known cause of senescence. They then treated a group of these cells with H-151, a compound that was previously noted to block STING [5].
The compound was found to be effective, as it decreased the gene expression of many pro-inflammatory factors, such as interleukins IL-6 and IL-8 along with CCL2 and CXCL10. However, it did not decrease these factors to the level of cells that had not been irradiated in the first place.
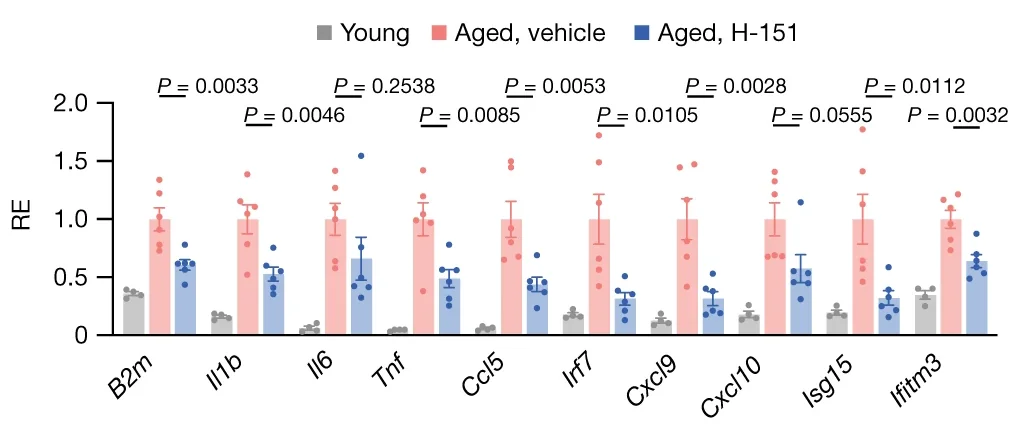
In a further experiment, the kidneys of young mice were compared to the kidneys of aged mice that had, and had not, received H-151. The results here were similar to that of human cells: interleukins, CXCL variants, and tumor necrosis factor (Tnf) were all substantially reduced. The same was found to be true for animals that had been genetically modified not to express STING.
Significant physical effects
The downstream effects of blocking STING in mice were clear. Whether older mice had been treated with H-151 or genetically modified, they were able to perform better on strength, endurance, and memory tests, including the well-known Morris water maze test.
A closer look at the brain cells revealed why. The treated or genetically modified mice had fewer overactivated microglia and more neurons in the hippocampus, and in this way, their brains were substantially closer to those of young mice than their aged, untreated, counterparts.
A further experiment confirmed the role of mitochondria DNA in activating STING within cells, and yet another line of experimentation used mice that had been genetically modified to express cGAS when triggered to do so. Triggering cGAS in these mice caused their neurons to die, with correspondingly worse performance on cognitive tests. This research confirms that the cGAS-STING pathway is indeed the culprit.
Is there a solution?
It is not clear in this study whether or not H-151 could be developed or modified into a drug that can proceed through the clinical trial process. There has been previous research into cGAS inhibitors that can disrupt this pathway; although such work was focused on autoimmune diseases and cancer [6]. As it appears that this pathway is a large part of brain inflammaging and accompanying cognitive decline, we look forward to a clinical trial of a drug that can target it.
Literature
[1] West, A. P., Khoury-Hanold, W., Staron, M., Tal, M. C., Pineda, C. M., Lang, S. M., … & Shadel, G. S. (2015). Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520 (Apri (7548)), 553–557.
[2] Ablasser, A., & Chen, Z. J. (2019). cGAS in action: Expanding roles in immunity and inflammation. Science, 363(6431), eaat8657.
[3] Dou, Z., Ghosh, K., Vizioli, M. G., Zhu, J., Sen, P., Wangensteen, K. J., … & Berger, S. L. (2017). Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature, 550(7676), 402-406.
[4] Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., & Santoro, A. (2018). Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology, 14(10), 576-590.
[5] Haag, S. M., Gulen, M. F., Reymond, L., Gibelin, A., Abrami, L., Decout, A., … & Ablasser, A. (2018). Targeting STING with covalent small-molecule inhibitors. Nature, 559(7713), 269-273.
[6] Li, Q., Tian, S., Liang, J., Fan, J., Lai, J., & Chen, Q. (2021). Therapeutic development by targeting the cGAS-STING pathway in autoimmune disease and cancer. Frontiers in Pharmacology, 12, 779425.