After three years in digital format due to COVID concerns, one of the oldest longevity conferences was again welcoming attendants in New York City. Ending Age-Related Diseases (EARD), organized by Lifespan.io for the sixth time, kept with modern ideas by using a hybrid format that combined in-person attendance with full online access in real time. This enabled the online participation of hundreds of experts and enthusiasts who could not travel to NYC in person.
This wasn’t the only aspect in which EARD-2023 led the way. The longevity space is changing, and we at Lifespan.io are trying to be at the forefront of those changes. As we see it, one of the most profound shifts, although still in its early stages, has been the introduction of decentralized science, or DeSci.
DeSci provides a vibrant and exciting alternative to the traditional framework of scientific discovery and translation, improving incentives and removing roadblocks. A pioneer of crowdfunded science, Lifespan.io has been very bullish about DeSci. We have collaborated with several DeSci organizations and initiatives, and so we have finally decided to bring DeSci to the main stage at EARD2023, officially naming it the Longevity + DeSci Summit.
While our previous conferences were mostly about science, this time, we fully embraced our advocacy side. There is a growing understanding in the field that advocacy is an integral part of the longevity ecosystem, indispensable for shifting public opinion and creating a favorable regulatory and investment climate.
We hosted our conference at Capitale, a historic building and the former seat of the Bowery Savings Bank, which retains a lot of its in-your-face grandeur. Turn-of-the-century interiors, complete with the bank vault and the lever-operated elevator, provided a refreshing contrast to the cutting-edge science being discussed on the stages and sidelines.

Inside the Capitale building
Unfortunately, we can only bring you a roundup of some of the talks from this extremely fast-paced and busy conference, which took place on two stages simultaneously, but photos are available now and videos will become available at some point.
Off to a good start
Lifespan.io president Keith Comito commenced the conference with a fast-paced overview of trends in citizen and decentralized science. He began with the opening of the first citizen science laboratory in the world, Genspace, in NYC in 2010, and then continued with Lifespan.io founding in 2014 along with our crowdfunding projects for SENS Research Foundation and other influential players in the longevity field. He noted how anti-aging research is being accelerated with novel tools and forms of organization, such as cryptocurrency, blockchain, and DAOs.
Keith made an important mention of earlier citizen initiatives, such as the Jimmy Fund, the massive grassroots campaign by Mary Lasker and Sidney Farber that elevated cancer research to the forefront of the US public agenda. We still have a lot to learn from those epic campaigns of old.
Keith then moved on to Zuzalu, the first-of-its-kind pop-up city that, earlier this year, brought together hundreds of people from several overlapping fields, including crypto, DeSci, and longevity, for two months of cohabitation and collaboration. Our team had boots on the ground in Zuzalu, and you can read all about it in our extensive coverage.
Briefly glancing over the improving attitudes towards longevity in society, Keith continued with an overview of Lifespan.io and its history and contributions to the field.

History, however glorious, is still history, and Keith backed it up with plenty of exciting news to report. First, our collaboration with Chris Hemsworth’s fitness app and community Centr, which turned to us to provide millions of its users with quality longevity-related content. We are proud of this opportunity, which can serve as a blueprint for extending the reach of longevity advocacy.
Keith also reported on the creation of the first ever longevity-related philanthropy NFTs. Those NFTs, based on the ancient image of Ouroboros, the dragon-snake biting its own tail, are automatically enhanced when their owner donates to longevity-related causes, and are “demi-soulbound” – that is, they are still tradable, but their upgradable aspects are based on the donations of the current holder. This is yet another creative way to boost awareness and philanthropy in our field.

But creativity never sleeps. Keith announced yet another collaboration, this time with the game development company Skillcap Studios on a game based on the Fable of the Dragon-Tyrant by the philosopher Nick Bostrom. The fable allegorically depicts aging as a cruel dragon that had been demanding multitudes of sacrificial victims from people until they acquired enough strength and knowledge to revolt against him. According to Keith, the “play-and-earn gaming incentivizes players to be healthy and builds up donations to fund research which could literally save them in real life.” The game is expected in the second quarter of 2024.
Why DeSci?
Todd White, steward of VitaDAO, expertly argued a case for decentralized science. He recounted the well-known problems that “traditional” science has been mired in, such as publication bias.
Todd highlighted misaligned incentives, including the “publish or perish” paradigm, which leads to the kind of publication bias that pushes researchers towards a wide variety of conscious and subconscious actions that pursue a single goal: to get published whatever it takes.
In this system biased against risk, failure is not rewarded. However, the null hypothesis, Todd said, is not a failure devoid of value. It is an important piece of information that should be made available to the scientific community – which the current system often prevents.
The DeSci movement is well positioned to alleviate, if not solve, many of those problems by reimagining the system of incentives and making research more open and collaborative.
For instance, intellectual property stored as an NFT (IP-NFT), one of the pillars of DeSci, is not a gimmick but a useful feature that ensures all the information about a project is kept “on-chain”, verified and reviewed, and is an asset in itself. This de-risks the first steps in bringing scientific knowledge to the market.
Todd talked about The Longevist, a website launched by VitaDAO that publishes curated preprints. Preprints that have received the most votes from the community are highlighted. The curating team includes big names, such as Andrea Maier and Matt Kaeberlein. The Longevist positions itself as an alternative to scientific journals that act as “profit-hungry intermediaries”.
Todd also touched upon the topic of longevity advocacy. “The communication the value of longevity has not reached maturity yet,” he said. “When researchers try to explain the field, they think about nuances, but people want soundbites.” This echoes Lifespan.io’s long-held views and relates well to our constant effort to hone our messages and the art of longevity advocacy as a whole.
Nature and longevity interventions work in different ways
One of the star guests of the conference was Vadim Gladyshev, a renowned Harvard geroscientist. In his talk, he started from the basics, by stating that there is still no agreement among the scientific community on what aging is. Different answers to this question, according to Vadim, lead to different strategies in fighting aging. Amazingly, with all the “geroscience boom” going on, there is not a lot of research into this fundamental question. “We need to agree on what we are optimizing”, Vadim said, “less chances of dying, fewer diseases, better function, or something else?”
While we can operate on all those levels, it is important to address the aging processes themselves. Vadim’s team developed a research program in order to address them directly. One of its parts attempts to understand what gives longevity to various systems, be it a cell type or an animal.
The second pillar is longevity interventions. While we know that some of them significantly extend lifespan in animal models, the question is if longevity of cells, longevity of organisms, and longevity-extending effects of certain interventions work along the same lines.
The third component is rejuvenation, the idea of reducing the biological age of an organism. It is different from aging, since a change from young to old is not necessarily the change from old to young, only in reverse. Questions of rejuvenation hence must be addressed separately. Working in all three directions will allow scientists both to slow and to reverse age-related changes.
Studying long-lived animals, Vadim’s team has discovered that the pathways leading to their exceptional longevity can differ between species. In a recent paper published in Cell, the group analyzed 41 species of mammals at an organ level to identify longevity signatures: omics-based patterns that illustrate the potential to live longer across mammalian species. The known maximum lifespan of a species is strongly correlated with patterns in gene expression, which provides a pathway towards longevity interventions.
Interestingly, “longevity signatures” across species do not correlate with the effects on gene expression produced by interventions. This means that the best interventions we have today, such as caloric restriction or rapamycin, work in different ways than those found by nature to extend maximum lifespan in long-lived species. This vast potential (after all, the difference in maximum lifespan across mammalian species is 100-fold) is “completely untapped”, according to Vadim, and longevity signatures provide “a direct tool for developing new interventions.”
It’s time to talk about female reproductive aging
After becoming a contributor to VitaDAO, Laura Minquini thought the model could be used to promote a highly important yet unfairly overlooked part of the longevity field – female reproductive aging. To turn things around, Laura created AthenaDAO, “a decentralized community to fund, govern, incubate, and support translational research into women’s health.”
Reproductive aging remains vastly under-researched, despite being relevant to half of the world’s population and to organismal aging in general. Beyond oncology, just 1% of healthcare research and innovation budget is spent on female-specific conditions. According to Laura, in longevity biotech investments, reproductive longevity comes second last, just before pets.
Decentralizing research into female reproductive longevity, Laura said, will empower women to invest directly into the topic that is highly relevant to them, circumventing the traditional avenues that apparently de-prioritize their plight. However, it is not only about research but also about education, advocacy, and access to resources. The aspiration, according to Laura, is to create a global female-led movement.
Formed only a year ago, in August 2022, AthenaDAO had already issued one IP-NFT to fund a research project on diminishing ovarian reserves. Six research projects are under advanced review as well as one stealth company. AthenaDAO prides itself on being the second biggest bio-DAO after VitaDAO.
Laura’s pitch to biotech is “Give women what they need, and they’ll become your best customers”. The menopause market, she said, is evaluated at 600 billion dollars, and yet research into menopause and its effects on the female organism is scarce. For instance, there are no biomarkers for when menopause begins (premenopause).
From the conference’s stage, Laura addressed the scientific community with a call for collaboration in several areas such as mitochondrial dysfunction, inflammaging, and the role of extracellular matrix in uterine pathologies.
Lifespan.io – an ecosystem-creating community hub for the field
The second day of the conference started with a keynote talk from Lifespan.io executive director Stephanie Dainow. “There are a lot of longevity conferences going on,” she said. “What we tried to be exceptional at is expanding and diversifying our audience. We have people from many different communities here: crypto, DeSci, government, and even general public, because we need to build the community.” Here are some excerpts from Stephanie’s talk:
Whether you are a transhumanist who wants to upload their mind, it doesn’t matter, because the first step we must take is to understand aging, and to do that, we need more funding, knowledge, collaboration, we need a united voice, so we can operate together more effectively.
To amplify the great work the scientific community is doing, we need precision and thoughtfulness in our messages, which Lifespan.io embodies. For a decade, we have been known for our unwavering dedication to responsible and high-quality longevity-related content, and our deep engagement with diverse audiences. We now have hundreds of thousands of monthly visitors to our website, and millions of subscribers across our various YouTube channels. We are a source of education and inspiration. We are bridging the gap between the research and the public, having become one of the foremost non-profits in the longevity domain.
We are architects of connection, introducing organizations and individuals to each other and actively supporting collaborations. The recent rapid expansion of the longevity field generated a clear need for a roadmap. We are guardians of progress, equipping scientists, investors, policy makers, and all community members with the tools they need to navigate this complex landscape. Our purpose is to facilitate cross-pollination, sparking those invaluable serendipitous connections. An embodiment of this is our Longevity Investors Network.
We have not yet reached the level of impact that was characteristic of the campaigns of old that fueled research into cancer and Alzheimer’s. In another example, climate change, once relegated to scholarly circles, now commands worldwide attention. Irrespective of differing viewpoints, we cannot deny the remarkable impact achieved. We need to recreate this success in our field.
Every interaction is an opportunity to kindle a flame, to attract fresh champions to our mission. Yet, the message must be meticulously crafted. We at Lifespan.io possess the expertise to shape the discussion, to counter the misconceptions. We tread this delicate line between exciting and overpromising. Collaborative problem solving, tolerance towards each other’s views, deduplication of efforts and effective resource allocation – Lifespan.io is a hub that supports all of this.
Making old drugs young again
Brian Kennedy, professor of biochemistry and physiology at the National University of Singapore and a star geroscientist, delivered a captivating update on his and his team’s work.
One of the problems with clinical trials in medicine is what Brian calls “the sickcare approach” which prioritizes treating symptoms of the disease after they appear over preventing them from appearing in the first place. In part, this stems from the Hippocratic Oath being reduced to one of its postulates, “do no harm”, which can be a road to hell paved with good intentions.
This entrenched approach makes it harder for the medical establishment to accept the need for healthy longevity studies. “We can’t work on someone who’s not sick”, many healthcare professionals would say. But doing nothing, Brian argued, is actually harming people. “This paradigm needs to be completely revisited,” he said. “Waiting until people get sick in their 50s and 60s is one of the least effective strategies you can take. You’re waiting till the problem is difficult before trying to solve it.”
Brian gave an overview of the studies his group is conducting on various potentially life-extending small molecules. Since even in mice, lifespan studies take a long time and are very expensive, the team has moved towards using biomarkers of aging and function in studies six to eight months long. This also allows for better histological analysis. “A complete change of strategy”, Brian called it.
The group pours additional resources into studying several doses rather than one arbitrarily chosen dose and making sure that both sexes are adequately represented, which is important given significant sex-related differences in aging (and, therefore, in the effects of anti-aging therapies).
Among the molecules currently being tested, Brian highlighted gemfibrozil, which reduced frailty in mice by 45%. Using a novel technique, the group was able to prove that previous research has misidentified gemfibrozil’s target as PPAR. The group has identified three true targets of gemfibrozil.
With another promising compound, urolithin A, the group saw a significant reduction of frailty in mice and “a very robust extension” in lifespan, although just in flies and killifish for now.
Many of the drugs Brian’s team is working on have been in use for decades, but they apparently can be repurposed as anti-aging drugs. Brian called this approach “making old drugs young again”.
Brian highlighted the importance of the combination approach in longevity medicine, and the fact that we are still not well-versed in creating such combinations. “When we combine things in mice,” he said, “we mostly get nothing – i.e., the effect size of the best single intervention. In many cases, the drugs actually cancel each other out. A cumulative effect is only the third most probable outcome”.
Mammals, unite!
At the beginning of his talk, Steve Horvath, “the father of methylation clocks” and a principal investigator at Altos Labs, outlined a major challenge in geroscience: the need for high quality data for AI-powered research and for full access to this data.
Thankfully, as a step in the right direction, the Mammalian Methylation Consortium founded by a group of scientists including Steve himself, just released data from more than 15,000 samples taken from 70 tissue types of 348 mammalian species. The data and the software to explore it are widely available, and in his talk, Steve called on anyone interested to put it to good use. Today, the Consortium includes more than 200 collaborators, an unprecedented number for our field.
According to Steve, a good biomarker of mammalian aging should be applicable to all mammals, because, in his opinion, all mammalian species age, even those who are sometimes credited with “negligeable senescence”.Steve’s group has even been able to develop an epigenetic clock for the naked mole rat. Most of Steve’s talk was dedicated to this particular statement.
To build this multi-species clock, the group had to work with tens of thousands of methylation sites that are highly conserved in mammals. This allowed them to build what Steve called the third-generation epigenetic clock.
Steve introduced the concept of relative age, which works like this: if the maximum lifespan in rats is four years, a two-year-old rat’s relative age would be 0.5, the same as the relative age of a 60-year-old human. Third-generation clocks predict this relative age based on methylation.
However, is it really possible to construct a clock that would be applicable to every mammalian species, without exception, given that mammals are separated by as many as 200 million years of evolution and their maximum lifespans differ 100-fold?
The answer, according to Steve, is yes, and the proof was recently published in Nature Aging. This new clock achieves remarkable correlation across all species, which, Steve said, shows that “something is deeply conserved about all those species, as if there is some clock ticking.” Here is how well this single clock applies to different mammalian species with vastly different maximum lifespans:
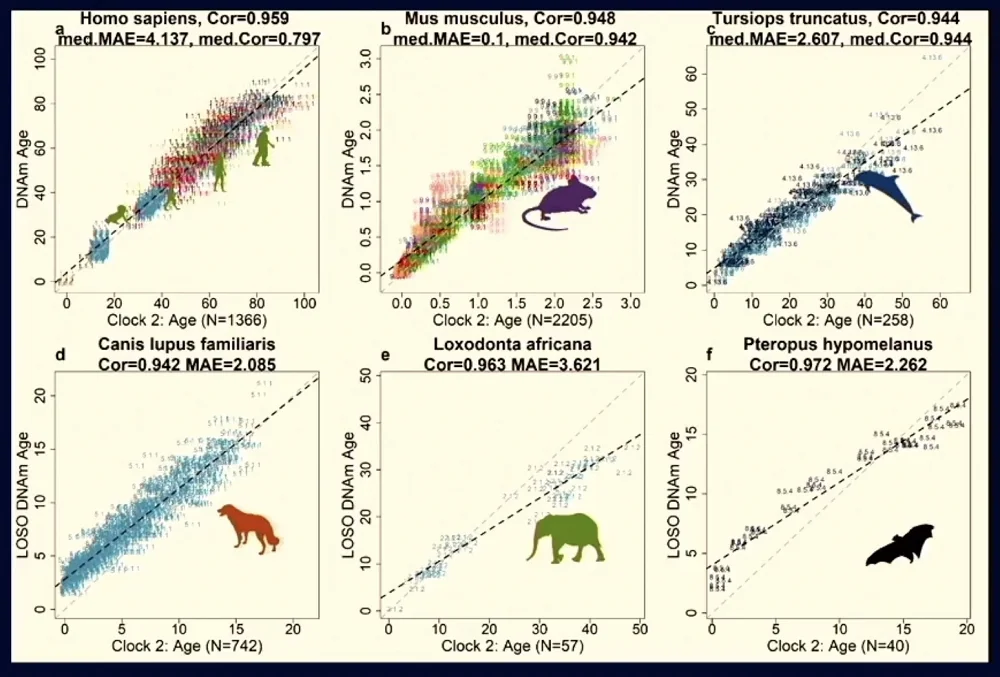
The new clock has been validated in vitro, showing an almost complete “wipeout” of epigenetic age following cellular reprogramming in human fibroblasts. Known anti-aging and pro-aging interventions (such as caloric restriction and high-fat diets, respectively) move the needle of the new clock in expected ways.
According to Steve, one of the inferences of this remarkable cross-species robustness is that if an intervention moves the needle in one mammalian species, it will probably do the same for other species, including humans.
Analyzing this pan-mammalian methylation data revealed yet another interesting finding: methylation signatures of popular anti-aging interventions do not match those associated with maximum lifespan. This suggests that while those interventions can make individuals live healthier and longer, they can hardly extend their lifespan beyond the maximum one for their species, which is consistent with a lot of previous research. To extend human maximum lifespan, new, currently undiscovered interventions are probably needed.
Can it even be done? Steve said that maximum lifespan is strongly correlated with the rate of change in methylation. Probably, anything that drastically slows this rate of change in humans has the potential to affect our maximum lifespan.
Finally, Steve reported that across multiple mammalian species, the rate of change in methylation is higher in young animals than in old ones. This interesting finding suggests that methylation is linked to development: starting from the moment of the embryonic reset, we basically begin to age rapidly, even though it will take decades for aging to manifest itself as dysfunction.
The two types of aging
In an interesting counterpoint to Steve Horvath’s talk, Peter Fedichev, CEO of Gero, gave a talk with the inspiring title of “How to Stop Worrying and Halt Aging”. However, the contents of the talk might have been less optimistic than the title.
A while ago, Peter, a physicist-turned-geroscientist, got the longevity field’s attention with his theory of aging, which postulates that humans and mice age fundamentally differently, even though mice are the most popular animal model in geroscience. In mice, dysregulation starts creating damage early on, and this damage becomes a runaway train, leading to a short maximum lifespan. In humans, remarkably long-lived species, potent regulatory mechanisms maintain homeostasis for a long time, but when they themselves become dysregulated, this is the point when we start experiencing accelerated aging.
Two major upshots from this theory are that mice are probably a bad animal model for discovering anti-aging interventions for humans and that slowing or even stopping aging in humans is fundamentally easier than reversing it (“true rejuvenation”). Peter’s theory, while far from being confirmed or even widely accepted, is interesting and worth keeping in mind. Read our recent interview with Peter, where it is explained in much more detail.
Is a super-drug coming?
Maxwell Biosciences, although not a household name in the longevity field, might be one of the most interesting companies in it. J. Scotch McСlure, its CEO, has a lot of experience and a fascinating personal history, as he got into biotech to save his father and daughter from disease. His company’s idea is to create a “synthetic immune system”.
Maxwell is currently working on a molecule with a wide variety of effects: it is effective against viruses, fungi, and bacteria, and it also has anti-cancer properties. Maxwell was initially funded by DARPA to create a “synthetic immune system”. It started with the peptide LL-37, which was discovered in heterochronic parabiosis studies. According to McClure, this unassuming molecule serves as an “orchestra conductor” for the entire immune system by recruiting immune cells and facilitating tissue repair and cleaning. Maxwell has been able to create a molecule that mimics LL-37 and is currently taking it to clinical trials.
Maxwell has received both government and private funding and conducted extensive preclinical studies that confirmed the molecule’s effectiveness against all tested enveloped viruses, drug resistant fungi, and more than 70 bacterial strains. Maxwell is collaborating with the Barron Lab in Stanford and Kirshenbaum Lab NYU, which pioneered this research about two decades ago.
LL-37 is a part of the “healing secretome”, as it is secreted by many tissues and universally present across the body. However, in vivo, it breaks down unpredictably and quickly, which precludes its use as a drug. The solution found was quite ingenious: to replace the carbon-based backbone of the peptide, vulnerable to degradation by protease enzymes, with a nitrogen-based one while leaving all the side chains intact. This modification also reduced the molecule’s size to the point that it is regulated by the FDA as a small molecule.
Maxwell has chosen infectious diseases as its indication, which allows for a much quicker and cheaper FDA approval process compared to fields like cardiovascular diseases or cancer. However, their discovery is potentially highly relevant to the aging processes, and we will probably be hearing about Maxwell a lot in the coming years.
We would like to thank our wonderful sponsors:
Platinum
Gold
Silver
Bronze