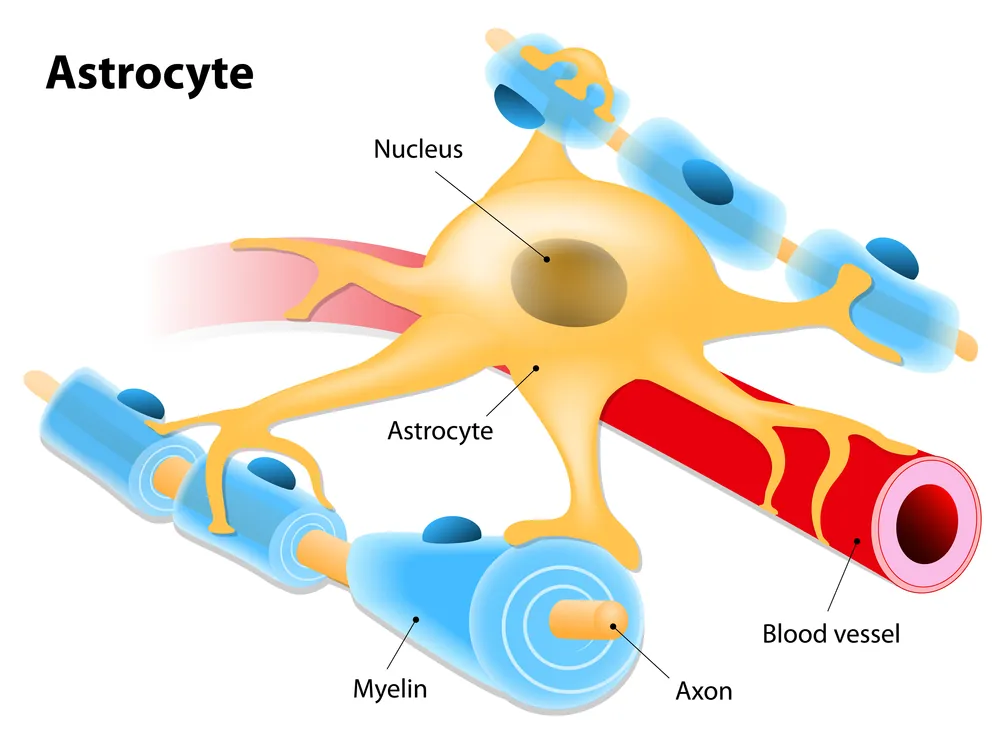
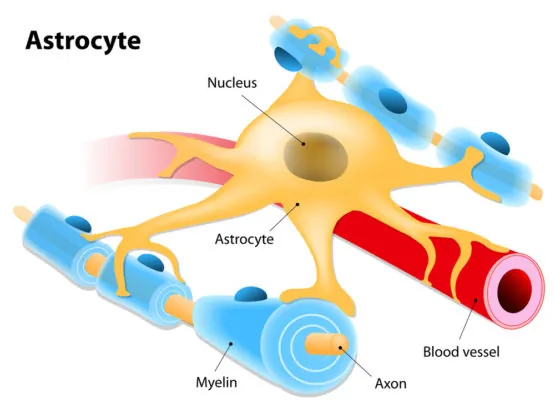
Researchers from the Salk Institute have discovered that a protein called Chrdl1, secreted by astrocytes, is responsible for driving synapse maturation and limiting brain plasticity later in life [1].
Abstract
In the developing brain, immature synapses contain calcium-permeable AMPA glutamate receptors (AMPARs) that are subsequently replaced with GluA2-containing calcium-impermeable AMPARs as synapses stabilize and mature. Here, we show that this essential switch in AMPARs and neuronal synapse maturation is regulated by astrocytes. Using biochemical fractionation of astrocyte-secreted proteins and mass spectrometry, we identified that astrocyte-secreted chordin-like 1 (Chrdl1) is necessary and sufficient to induce mature GluA2-containing synapses to form. This function of Chrdl1 is independent of its role as an antagonist of bone morphogenetic proteins (BMPs). Chrdl1 expression is restricted to cortical astrocytes in vivo, peaking at the time of the AMPAR switch. Chrdl1 knockout (KO) mice display reduced synaptic GluA2 AMPARs, altered kinetics of synaptic events, and enhanced remodeling in an in vivo plasticity assay. Studies have shown that humans with mutations in Chrdl1 display enhanced learning. Thus astrocytes, via the release of Chrdl1, promote GluA2-dependent synapse maturation and thereby limit synaptic plasticity.
What is brain plasticity
The brain was once thought to be an unchangeable organ that stayed the same throughout your life. In reality, the brain can reorganize itself throughout your life, although this ability does appear to diminish as you age. The brain’s ability to rewire itself, creating new neurons and new connections between them while purging older, unnecessary connections, is known as brain plasticity or neuroplasticity; it is absolutely essential in allowing us to learn new things, form memories, and repair damaged brain tissue, and, not surprisingly, it’s at its peak when we are very young and our brains are still developing.
The extent to which brain plasticity decreases as we age is still a somewhat controversial matter; in any case, ways to enhance the brain’s plasticity may be needed both for therapeutic reasons and to allow us to continue to learn new things if rejuvenation therapies grant us very long lives.
The study
Salk researchers set out to find out the role that a type of brain cell called an astrocyte has in brain plasticity; not much is known about the role of astrocytes in the adult brain, and in order to learn more about them, the authors of the study decided to focus their attention on a specific protein secreted by these cells, namely Chrdl1, or chord-like 1.
It is known that synapses—structures that allow neurons to communicate—transition from an immature to a mature state when certain calcium-permeable receptors on their surfaces are replaced by calcium-impermeable receptors; this change is what allows synapses to become stable. In their study, the researchers showed that this process is regulated by astrocytes through the secretion of Chrdl1.
To figure it out, the researchers engineered mice that were unable to produce the protein by switching off the gene that encodes for it. The result was that, even as adults, these mice had a much higher level of plasticity than normal adult mice; in fact, the Chrdl1-knockout mice exhibited immature synapses and levels of plasticity comparable to those of young mice. Furthermore, the researchers didn’t just show that the protein has some effects on synapse maturation; according to the study, the expression of the protein is both necessary and sufficient for the maturation of synapses.
Conclusion
We don’t know much about the role of the Chrdl1 protein in the human brain, although some studies had shown alterations in patients suffering from schizophrenia and bipolar disorder.
If brain plasticity never decreased at all, this would prevent our brains from stabilizing and would force them in a sort of childlike state for our entire lives; however, being able to restore more youthful levels of plasticity may prove extremely useful for the sake of restoring lost connections in areas of the brain that have been affected by conditions such as stroke.
Literature
[1] Blanco-Suarez, E., Liu, T.-F., Kopelevich, A., & Allen, N. J. (2018). Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron.