Steve Horvath, Vera Gorbunova, Alejandro Ocampo, and their team have used partial reprogramming to repair DNA damage in a mouse model. They published their findings in Frontiers in Aging.
Building a path to rejuvenation
Read More
The researchers begin their paper by discussing these four well-known factors along with the concept of partial cellular reprogramming, which resets the cells’ epigenetics, sending them back into a functionally younger state. Substantial prior work has demonstrated that partial reprogramming mitigates multiple hallmarks of aging in living animals [1]. Both protein machinery and RNA transcription has been affected in animal models [2, 3].
However, only limited work has focused on epigenetic reprogramming’s effects on base genomics and the ability of cells to repair their own DNA, particularly when the cells have already sustained significant damage. To that end, the researchers used a mouse model of progeric, rapid aging that lacks the vital repair factor Ercc1.
A greater response
Fibroblast cells from the tail tips of the Ercc1-lacking mice, as well from mice that had normal DNA damage repair abilities, were tested for the DNA damage marker γH2AX. As expected, the Ercc1-less mice had increased amounts of this marker, and their cells were enlarged as well.
All of these animals were genetically engineered to express the OSKM factors in the presence of doxycycline. Some animals were only administered this compound, while others also received Vitamin C, which lowers natural barriers to epigenetic reprogramming, along with an activator of the Wnt pathway, which affects cellular proliferation and development.
Samples from both Ercc1-depleted and more ordinary animals had positive responses to OSKM treatment, particularly alongside the other two compounds. The epigenetic age of one sample was reduced by more than half after four days. Heterochromatin, which is increased in Ercc-less cells as a response to damage, was restored to the levels of ordinary cells, as was γH2AX and nuclear size. This coincided with the epigenetic reset, leading these researchers to concur with previous work [1] that there is a causative relationship involved.
Most interestingly, the Ercc1-depleted cells showed a stronger epigenetic response after reprogramming than the cells from more ordinary mice. Vitamin C and the Wnt promoter (VC) were found to have a very significant effect on DNA repair, strongly upregulating various genes responsible for rejoining the ends of damaged DNA, along with fixing mismatches and missing DNA bases.
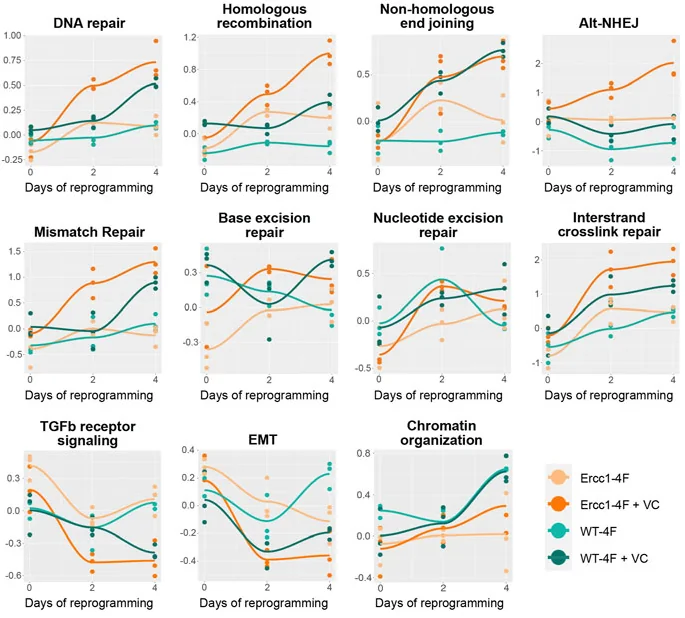
A potential shortcut to DNA repair
This study’s findings coincide with previous work showing that reprogramming inhibits the TGFb pathway, which is associated with aging phenotypes and whose inhibition has been found to extend lifespan in worms [4]. The researchers also examined the related bone morphogenic pathway (BMP).
In Ercc1-depleted fibroblasts, most of the inhibitors that affected either TGFb or BMP were found to also decrease γH2AX. The effective inhibitors also reduced nuclear size. Three inhibitors were also found to lead to significant decreases in epigenetic age. showing a strong link between TGFb inhibition, reprogramming, and genetic repair.
RNA sequencing confirmed these findings. Many of the gene sequences that were found to be upregulated and downregulated with TGFb inhibition were also affected in the same way at day 4 of reprogramming. The affected gene sequences spanned the range of cellular functions, including the extracellular matrix, other structural proteins, protein metabolism, and alcohol metabolism. However, some DNA repair processes that were enhanced with reprogramming were not enhanced with TGFb inhibition in Ercc1-depleted fibroblasts.
While performed primarily on cells derived from a heavily modified mouse model and certainly does not provide definitive evidence, this study provides guidelines for future research. If TGFb inhibition is found to be less dangerous than reprogramming, and can be conducted on living organisms without severe side effects, it may be possible to use this technique to reduce aging phenotypes and so ameliorate age-related diseases.
Literature
[1] Ocampo, A., Reddy, P., Martinez-Redondo, P., Platero-Luengo, A., Hatanaka, F., Hishida, T., … & Belmonte, J. C. I. (2016). In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell, 167(7), 1719-1733.
[2] Benevento, M., Tonge, P. D., Puri, M. C., Hussein, S. M., Cloonan, N., Wood, D. L., … & Heck, A. J. (2014). Proteome adaptation in cell reprogramming proceeds via distinct transcriptional networks. Nature communications, 5(1), 5613.
[3] Plath, K., & Lowry, W. E. (2011). Progress in understanding reprogramming to the induced pluripotent state. Nature reviews genetics, 12(4), 253-265.
[4] Schoenfeldt, L., Paine, P. T., Kamaludeen M, N. H., Phelps, G. B., Mrabti, C., Perez, K., & Ocampo, A. (2022). Chemical reprogramming ameliorates cellular hallmarks of aging and extends lifespan. bioRxiv, 2022-08.