A new study published in Aging Cell applied an artificial intelligence target discovery platform to aid in identifying potential dual-purpose targets for anti-aging and anti-cancer treatments [1].
Killing two birds with one stone
Aging, as is the case with many diseases, is a risk factor for cancer, and the processes behind aging and cancer are interconnected. Therefore, targeting their common molecular pathways may be effective against both conditions. This interconnection has been described in the literature: for example, some Hallmarks of Aging, which describe common age-related problems, are also frequently associated with cancer [2, 3].
Additionally, some gerotherapeutic drugs, which aim to decrease the burden of age-related diseases, are already FDA approved for cancer treatment [4, 5].
Therefore, the authors of the paper decided to build on that knowledge and “identify potential dual-purpose targets that could delay aging and increase lifespan among healthy individuals as well as inhibit cancer using a list of common cancer targets.”
The quest to find the dual-purpose targets
The researchers used gene expression data from 47 healthy tissues of 980 individuals to identify age-associated genes. They analyzed the differential expression between old and young people in order to build correlations between gene expressions and aging.
For cancer-associated genes, they “used 139 datasets (131 transcriptomics and 8 proteomics) consisting of 11,303 cases and 4431 control samples selected from 11 age-associated solid cancers.”
It would be a Herculean effort to manually review all the datasets containing thousands of genes and proteins, along with their interaction data, in order to identify common cancer and aging targets. Therefore, the researchers employed AI tools, specifically PandaOmics, an AI-driven target discovery engine that they previously developed [6].
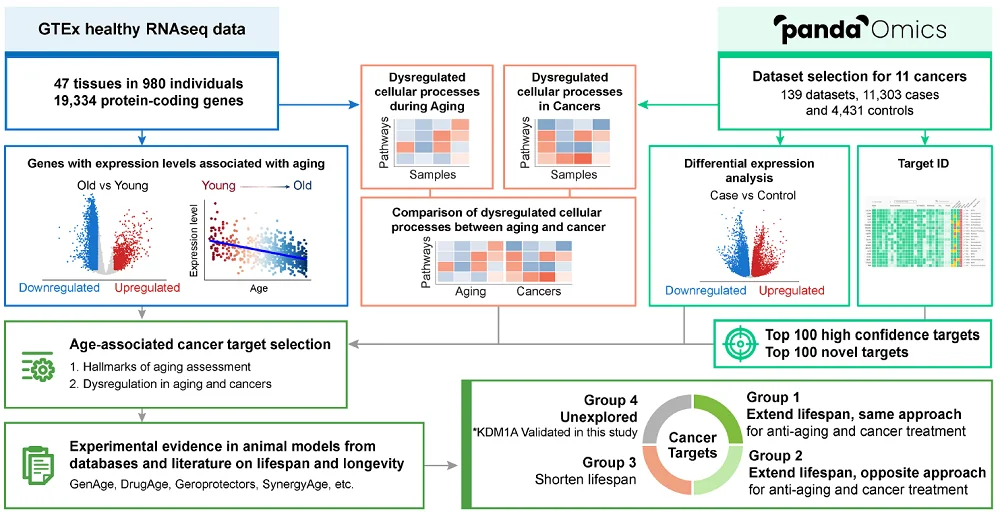
The researchers started their work by identifying druggable cancer targets. First, they focused on high-confidence targets that had been broadly discussed in the literature. Second, they looked for novel targets, which had fewer studies or were not well described in scientific literature.
The identified targets were further analyzed regarding age-related expression. In this way, the researchers identified 180 age-associated cancer targets, of which 119 were already associated with FDA-approved or clinical investigational drugs.
The researchers then searched multiple databases to identify which of the 180 age-associated cancer targets could also be potential anti-aging targets, separating them into four groups.
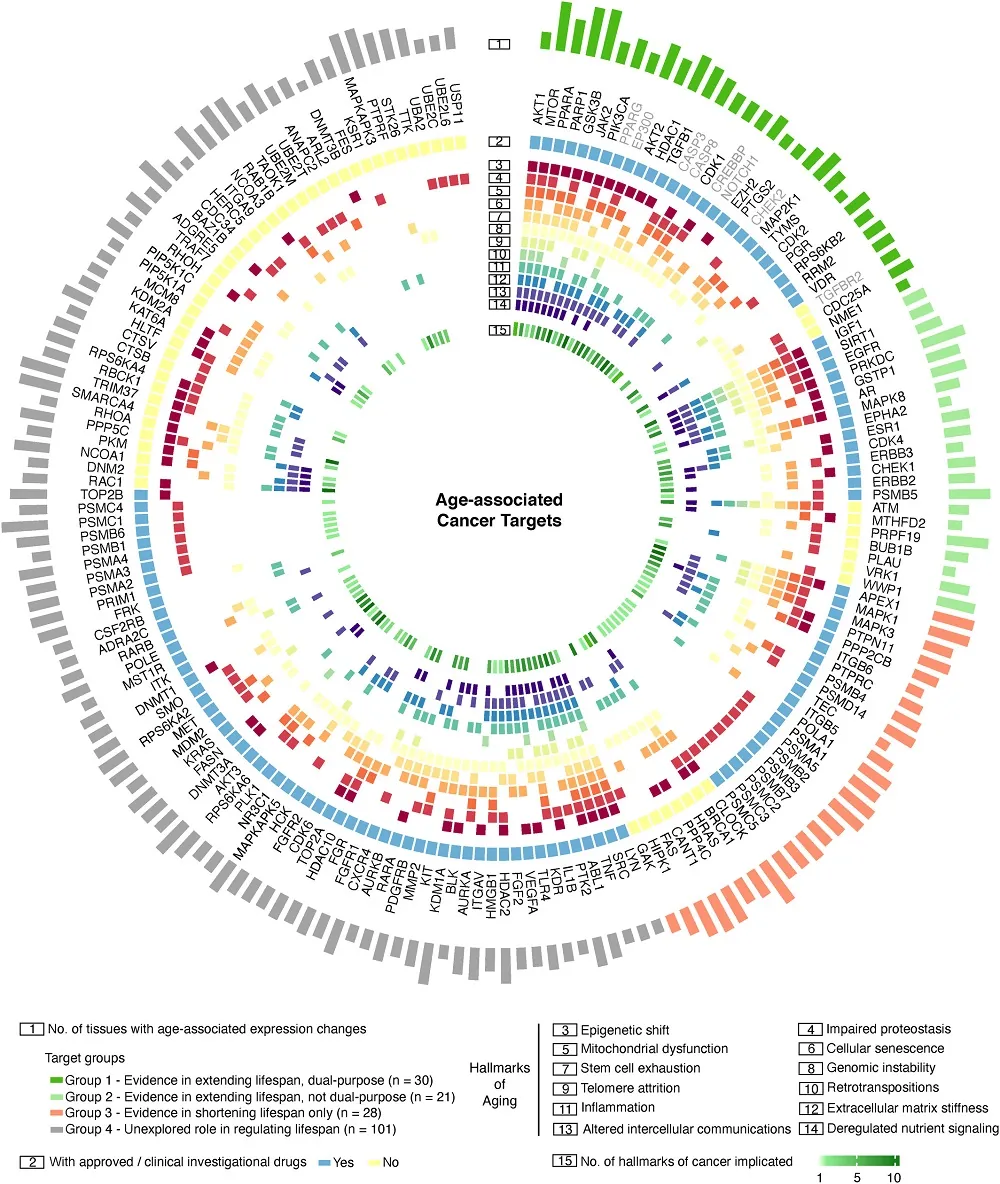
Group 1 consisted of targets described in the literature to have lifespan-extending properties. They were also characterized by the “same direction of therapeutic inhibition or activation for anti-aging and anti-cancer treatment.” Group 2 also consisted of targets described in the literature to be related to extended lifespans, but inhibiting or activating them for anti-aging would have potentially carcinogenic side effects. Group 3 consisted of targets that shorten lifespan, and Group 4 consisted of cancer targets that weren’t researched regarding lifespan extension
Analysis of the genes included in Group 1 revealed that the genes were likely to be involved in such processes as cellular senescence or other signaling pathways. The researchers also looked at such things as mechanisms of action, clinical trial status, relation to the hallmarks of aging, and role in modulating longevity, and used this information to propose therapeutic approaches. They noted that there already exist FDA-approved inhibitors for three of the targets they identified in Group 1, and five other targets are currently being investigated in clinical trials.
The researchers took a closer look at Group 4, which consisted of potential targets that haven’t yet been investigated in lifespan studies. The researchers performed an analysis comparing the enrichment of biological processes between Group 4 and Groups 1 and 2.
Combining this analysis with an eye towards mechanisms of action and roles in age-associated diseases, the researchers selected 23 potential dual-purpose targets. Just as in the case of the previous group, they proposed therapeutic approaches using those targets. The majority of the identified targets were associated with FDA-approved or investigational drugs.
One of the 23 predicted dual-purpose candidates, lysine-specific demethylase 1A (KDM1A) is involved in carcinogenesis [7], and its inhibitors are currently being tested experimentally [8, 9]. It was also dysregulated during aging in most tissues analyzed in this study. This prompted the researchers to analyze its effect on lifespan.
When a homolog of KDM1A was inactivated in C. elegans worms, it significantly increased the animals’ median lifespan by 15.8%, making it an interesting candidate for further testing as a dual-purpose target.
One drug, two targets
The authors of this study observed that cellular senescence-associated pathways were the most upregulated pathways in both aging and cancer tissues, suggesting that targeting those pathways might combat cancer and delay aging. In this light, the authors speculated about the role of senolytics in the dual-target strategy that they are proposing.
Senolytics have been shown previously to reduce senescence and have a positive impact on age-associated conditions [10]. Since senescence has been shown to promote processes associated with tumor growth [11], these observations suggest that senolytics can be promising candidates, which, through their impact on senescent cells, can lead to delayed aging and cancer treatment.
Senolytics and other molecules that target these identified genes would need to be further tested as potential dual-purpose molecules for treating cancer and aging.
Literature
[1] Pun, F. W., Leung, G. H. D., Leung, H. W., Rice, J., Schmauck-Medina, T., Lautrup, S., Long, X., Liu, B. H. M., Wong, C. W., Ozerov, I. V., Aliper, A., Ren, F., Rosenberg, A. J., Agrawal, N., Izumchenko, E., Fang, E. F., & Zhavoronkov, A. (2023). A comprehensive AI-driven analysis of large-scale omic datasets reveals novel dual-purpose targets for the treatment of cancer and aging. Aging cell, e14017. Advance online publication.
[2] Aunan, J. R., Cho, W. C., & Soreide, K. (2017). The biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging and Disease, 8(5), 628–642.
[3] Blagosklonny, M. V. (2022). Hallmarks of cancer and hallmarks of aging. Aging (Albany NY), 14(9), 4176–4187.
[4] Ballou, L. M., & Lin, R. Z. (2008). Rapamycin and mTOR kinase inhibitors. Journal of Chemical Biology, 1(1–4), 27–36. https://doi.org/10.1007/s12154-008-0003-5
[5] Populo, H., Lopes, J. M., & Soares, P. (2012). The mTOR signalling pathway in human cancer. International Journal of Molecular Sciences, 13(2), 1886–1918.
[6] Pun, F. W., Ozerov, I. V., & Zhavoronkov, A. (2023). AI-powered therapeutic target discovery. Trends in Pharmacological Sciences, 44(9), 561–572.
[7] Li, L., Wang, Y., Mou, Y., Wu, H., & Qin, Y. (2021). KDM1A identified as a potential oncogenic driver and prognostic biomarker via multi-omics analysis. Canadian Journal of Infectious Diseases and Medical Microbiology, 2021, 4668565.
[8] Fang, Y., Liao, G., & Yu, B. (2019). LSD1/KDM1A inhibitors in clinical trials: Advances and prospects. Journal of Hematology & Oncology, 12(1), 129.
[9] Karakaidos, P., Verigos, J., & Magklara, A. (2019). LSD1/KDM1A, a gatekeeper of cancer Stemness and a promising therapeutic target. Cancers (Basel), 11(12), 1821.
[10] Raffaele, M., & Vinciguerra, M. (2022). The costs and benefits of senotherapeutics for human health. Lancet Healthy Longev, 3(1), e67– e77.
[11] Collado, M., Blasco, M. A., & Serrano, M. (2007). Cellular senescence in cancer and aging. Cell, 130(2), 223–233.