Once a year, Copenhagen becomes a Mecca for the longevity community. Hundreds of people flock to the picturesque Danish capital to attend the Aging Research and Drug Discovery Meeting (ARDD) for five full days of talks by geroscientists and biotech leaders, mingling and networking with like-minded longevity enthusiasts, and not-so-healthy late night-outs.
ARDD is organized by Insilico founder and CEO Dr. Alex Zhavoronkov, Prof. Morten Scheibye-Knudsen of the University of Copenhagen, and their dedicated team of helpers who did a fantastic job putting this vast event together. Since 2019, ARDD has been held under the auspices of the University of Copenhagen, which provides its opulent Festival Hall as the main stage for the talks.
ARDD is one of the longest-running longevity conferences, and this was its tenth incarnation. According both to dry numbers and the consensus of the crowd, this year’s conference was the biggest and best of them all, hosting some 600 in-person participants and around ten times more online viewers.
It was also quite intense, with wall-to-wall talks, panel discussions, and poster sessions from 9 am to as late as 9 pm. In the final talk of the conference, Prof. Vadim Gladyshev of Harvard jokingly referred to those who had sat through all the 100+ talks as “centenarians” (just like with real centenarians, there were very few of them). Exciting and fascinating as all the talks were, we are only able to present a fraction of them here. Our apologies to those who were left out.
Proving the geroscience hypothesis
In the inaugural talk, the veteran geroscientist James Kirkland, director of the Robert and Arlene Kogod Center on Aging, gave an overview of some aspects of the longevity field, including ongoing trials (mostly senolytic-related) and the search for good biomarkers of aging. According to Kirkland, more than 80 clinical gerotherapeutic studies are underway, interventional and observational, but biomarkers that reliably react to interventions are needed, which cannot be said about all current tentative biomarkers of aging.
More broadly, Kirkland talked about recent research supporting the geroscience hypothesis, which predicts that aging processes start early in life and that targeting them should delay, prevent, and alleviate multiple diseases. We are seeing more and more of it – for now, mostly in pre-clinical models. As long as the underlying hypothesis is correct, we can hope to eventually find those elusive anti-aging treatments.
Among the recent studies mentioned by Kirkland were a 2021 study that showed great results for the senolytic quercetin against COVID-19 infection (quercetin drastically lowered hospitalization, need of oxygen, and need of ICU) and two studies suggesting a “1-2 punch approach” for cancer, when cancer cells that had gone senescent following an anti-cancer treatment are subsequently eliminated by senolytics.
So much is owed to so few MSCs
Thomas A. Rando, Deputy Director of Stanford Center on Longevity, invited the audience into the amazing world of muscle stem cells (MSC) and muscle rejuvenation in the context of exercise. Muscle’s high regenerative potential, which makes it a good model of tissue repair and rejuvenation, is mediated by a small number of normally quiescent muscle stem cells that enter a proliferation frenzy when needed. Age-related decline in this proliferation capacity results in slower regeneration, more scarring, and stiffer muscle tissue.
Rando reported on some of the recent work on rekindling muscle stem cells’ regeneration prowess. Exercise enhances aged muscle regeneration, and scientists are beginning to understand the molecular mechanisms behind this effect. For instance, exercise robustly upregulates the multi-role protein cyclin D1 in aged muscle stem cells. This negatively correlates with the expression of the cytokine TGFß, which plays a central role in inflammation. Both induction of cyclin D1 and nhibition of TGFß restore functionality of aged muscle stem cells.
Interestingly, exercise positively affects multiple types of stem cells, including neural stem cells. Its benefits are also seen in immune cells, such as muscle resident macrophages. This allows us to view muscle as an endocrine organ highly relevant to organismal aging. Rando also reported that plasma from exercised mice positively affects aspects of aging in age-matched non-exercised animals.
On day 2, Rando delivered a second talk on epigenetic control of stem cell aging and rejuvenation. Aging results in dysregulation of the methylation of histones, the proteins that chromatin is packed around. Muscle stem cells exhibit dense heterochromatin. Since chromatin-related enzymes use metabolites as cofactors, the epigenome is basically modified by metabolism. According to Rando, S-adenosylmethionine (SAM) is required for the methylation process, as it serves as a methyl group donor. Polyamines, such as spermine and spermidine, consume SAM, and their levels increase with age. Inhibition of polyamine synthesis restores histone expression and loss of heterochromatin, and supplementation with SAM rescues the regenerative potential of muscle stem cells.
The need for a strong grassroots movement
In contrast to previous strictly scientific talks, Michael Ringel, managing director and senior partner at Boston Consulting Group, talked mostly about the economics of longevity and the importance of advocacy and education.
Ringel began his talk with the largely undisputable maxim “Life and health are valuable”. Geroscience is a way to extend lifespan and healthspan for all, but amazingly, it’s “not yet on the public radar” – at least, not as the net positive thing it clearly is. To turn things around, Ringle said, the longevity field needs a strong grassroots movement.
Today, he continued, conventional medicine is close to the limit of its abilities to fight age-related diseases, where spending more money results in only minuscule gains in life expectancy. Even conquering all major degenerative diseases, a hardly achievable goal, can only get us so far. Targeting the underlying processes of aging instead, which is what geroscience is trying to do, seems the only way forward.
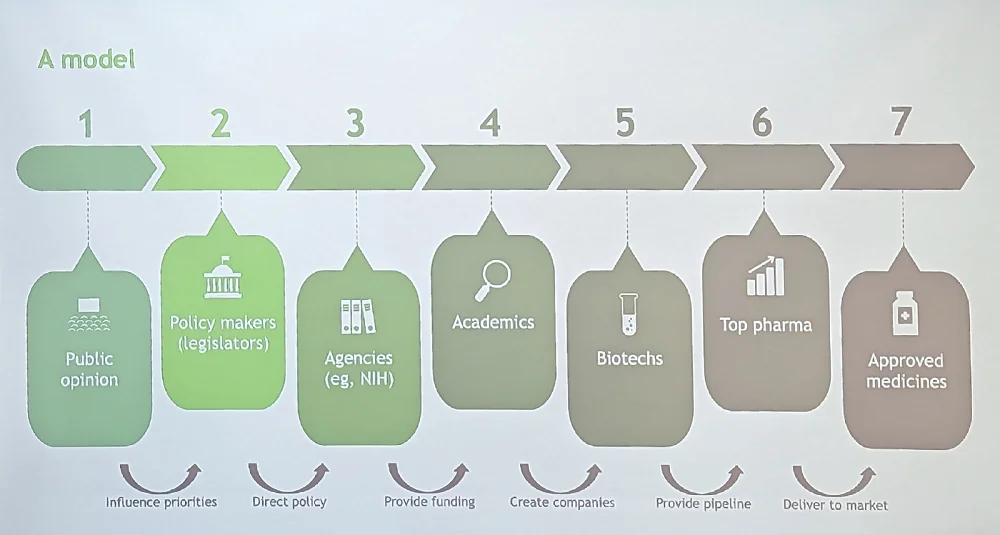
In one impressive slide, Ringel outlined his view of the process of taking anti-aging therapies to the market. This, according to him, should start with influencing public opinion, which, in turn, drives legislation and resource allocation. Having advocated for more longevity education and lobbying for years, we at Lifespan.io cannot agree more.
Ringel specifically mentioned two examples: Mary Lasker’s public awareness campaign, which led to the War on Cancer, and the more recent ALS Ice Bucket Challenge. The latter not only raised 220 million dollars in direct donations but also led to a four-fold increase in NIH funding, a 20% increase in research, and ultimately to three new FDA-approved drugs. Sadly, geroscience is still the stepchild of healthcare spending, with zero legislative initiatives, a meager 0.7 billion dollars in federal funding, and no FDA-approved drugs. The upside is that there is no way from here but up.
Prevention is the key
In his talk titled “From geroscience to gerotherapeutics”, Prof. Nir Barzilai of Albert Einstein College of Medicine echoed the previous speaker by pointing out that most of the increase in life expectancy humanity has achieved so far was due to prevention; hence, our goal is to prevent age-related diseases. To do so, future gerotherapeutics must target more than one hallmark of aging, as rapamycin does in animal models.
Since the first day of the conference was partly devoted to longevity medicine, Barzilai, himself a medical doctor, touched on the relationship between geroscience and physicians. “From geroscience to gerotherapeutics,” he said, “we are on the right path. However, our biggest challenge is that there are many ways to target aging, and demand is outpacing supply. Many people could already benefit from maximizing their health, and having more clinics and physicians will pave a brighter path for all”. Barzilai puts a lot of hope in educating physicians on geroscience, but admits that the principle “do no harm”, taken too literally, “makes MDs conservative” when it comes to adopting the geroscience paradigm.
Barzilai, the world’s foremost expert on metformin and the principal investigator in the upcoming much-anticipated TAME (Targeting Aging with Metformin) trial, gave an update on this drug. While things have sometimes been rough for metformin, with the Intervention Testing Program (ITP) failing to detect lifespan extension in mice, and one paper last year casting doubt on some previous assertions about it, Barzilai presented more optimistic data. According to him, people on metformin had half the mortality rate and hospitalization with COVID, which ties metformin to the hallmarks of aging.
Discussing the appropriate time for a healthy person to start taking metformin, Barzilai mentioned the antagonistic pleiotropy principle, which suggests that not all drugs that are good for you when you’re old are also good for you when you’re young, and metformin might be one of those drugs. The bottom line is that metformin might not be advised for younger healthy people, although this demands further investigation.
AI is the future
One of the conference’s hosts, Dr. Alex Zhavoronkov of InSilico Medicine, delivered an update on AI use for drug discovery. Zhavoronkov described Pharma.ai, an end-to-end drug discovery and development platform developed by InSilico that consists of three modules: Panda.ai for omics analysis and target and biomarker identification; Chemistry42 for de novo small molecule generation and virtual screening, and InClinico for clinical trial outcomes prediction. According to Zhavoronkov, Panda.ai is already used by hundreds of companies and scientific teams.
The era of generative AI is coming to geroscience, promising to speed up drug discovery even more while also saving a lot of money. This was made evident by some of the case studies Zhavoronkov presented.
InSilico is also an active educator, helping create some of the best online geroscience and longevity medicine courses around. In his talk, Zhavoronkov announced a new course on disease modeling and target discovery, consisting of seven lectures and now available on the company’s website.
Zhavoronkov was also visibly proud to present the new roboticized InSilico lab, looking straight out of a well-funded Hollywood sci-fi movie, thanks to elaborate ambient lighting and sliding doors.
However, the big announcement came a few days after the conference: apparently, while in Copenhagen, Zhavoronkov was working on finalizing a major deal with the pharma company Exelixis. The latter has licensed ISM3091, InSilico’s leading candidate drug for treating BCMA-mutated cancers, for 80 million dollars in addition to undisclosed milestone payments. The drug is currently undergoing Phase I trials.
Interestingly, among all the data-packed slides, Zhavoronkov also touched on the question of whether aging is a disease. In his view, it hardly matters in the context of pharmaceutical drug approvals, since “if the drug works in aging, it should work in a variety of diseases.” While there is truth in this, it is unclear to what extent this approach of hitting one disease at a time is limiting geroscience’s potential.
Longevity medicine in the spotlight
Longevity medicine, a budding field that aims at shifting the focus towards disease prevention in accordance with the geroscience principles, was on full display in Copenhagen. Prof. Tzipi Strauss reported on the world’s first longevity clinic affiliated with a public hospital, which opened in Israel earlier this year. Sheba Hospital has been on the list of ten best hospitals in the world for several years now, and it might be the perfect place for research into longevity medicine, as Strauss told us in her interview. Sheba is also a university research hospital affiliated with Tel Aviv University and running cutting-edge research.
The executive medical board of the new center boasts big names, such as Nir Barzilai and Alex Zhavoronkov. In her talk, Strauss listed “the four A’s”, the principles that will guide the center’s work: Accessibility, Affordability, Academic (research), and AI.
Israel’s healthcare system is among the best in the world, flexible and highly digitized, which should help the center to obtain various healthcare data and effectively run human trials. Tzipi reported on the upcoming SHARP trial (n=1500), the center’s first initiative, which will include several interventions that are rigorously tested using a battery of biomarkers.
Another prominent expert in the field of longevity medicine, Prof. Evelyne Bischof, announced at the conference her moving to Israel to co-pilot the center.
In her talk, Bischof reiterated the tenets of longevity medicine, including replacing the current “reactive medicine” that many people call “sickcare” with the prevention paradigm, which requires deploying methods novel to MDs such as multi-omic analyses. Longevity medicine starts with healthy longevity diagnostics and continues with developing highly personalized regimens. Bischof stated that it is imperative that high-quality longevity medicine eventually becomes available to all. Shealso gave a second, more science-oriented talk at the conference, centered on geroncology, where she argued for integrating longevity medicine into oncology decision making and guidelines.
Dr. Andrea Maier of the National University of Singapore announced the opening of another hospital-affiliated longevity clinic, this time in Singapore. The new clinic, which opened its doors on August 31st, is affiliated with Alexandra Hospital and collaborates with the longevity center in Sheba (both centers are also collaborating with Mayo Clinic).
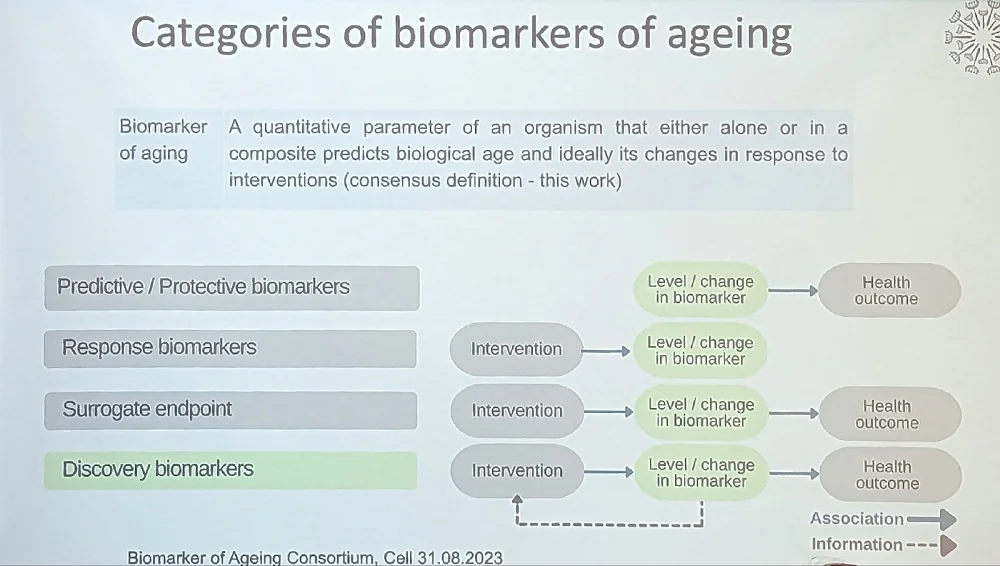
Stressing the importance of biomarkers of aging for longevity medicine, Maier also gave an overview of this topic based on this fresh-off-the-press paper by Biomarkers of Aging Consortium, of which she is a member.
Exercise and inflammaging
Bente Klarlund Pedersen, of the Center for Physical Activity Research at Rigshospitalet, Denmark, gave a talk on the anti-inflammatory effects of exercise.
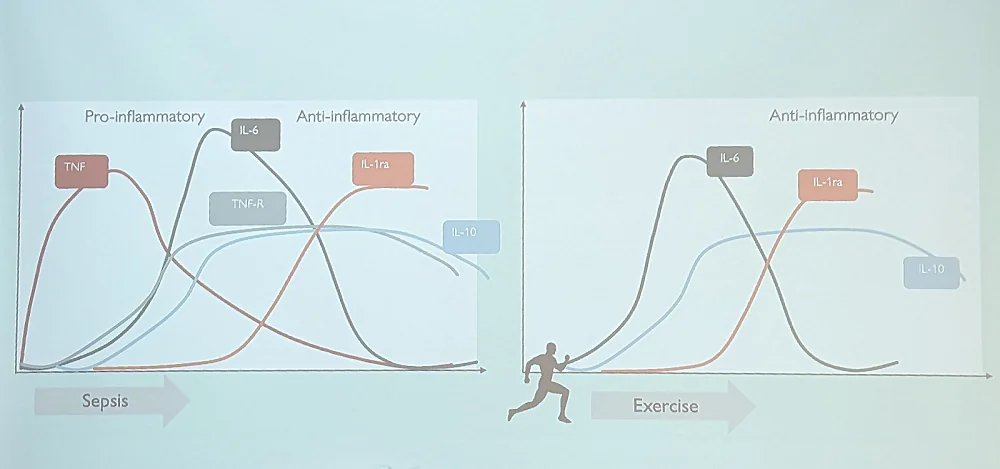
Inflammaging, the ubiquitous sterile age-related inflammation, might contribute to as many as 50% of all deaths. It is known that exercise lowers inflammation, but the actual story behind it is much more complex and fascinating. Bente described the “IL-6 paradox”: while high resting levels of this inflammatory cytokine are associated with obesity and physical inactivity, acute exercise also induces a marked increase in IL-6.
Apparently, IL-6 is released by muscle into the blood, which is followed by an increase in anti-inflammatory cytokines (sometimes up to 100-fold). This process is markedly different from sepsis, in which a similar spike in IL-6 is induced by an increase in another pro-inflammatory cytokine, TNF. While sepsis is basically an acute inflammatory response followed by an anti-inflammatory response, exercise induces a much healthier interplay of cytokines (probably by inhibiting TNF), which not just lowers inflammation but also increases glucose uptake, improving insulin resistance. Exercise also counters the increase in TNF induced by endotoxin.
Pedersen told the audience about a study in which healthy young males voluntarily and drastically decreased their daily physical activity for a period of two weeks, which led to impaired glucose uptake and insulin signaling, hyperlipidemia, loss of muscle mass and fitness, and an increase in visceral fat mass. The main takeaway is that physical inactivity induces chronic systemic inflammation (at least in part via macrophage infiltration triggered by visceral fat accumulation), while exercise lowers it.
How do you want your gene spliced?
Luigi Ferrucci from the National Institute on Aging gave a fascinating talk on a topic which clearly is not getting the attention it deserves yet: alternative splicing (AS), a molecular process that allows a single gene to produce multiple distinct messenger RNA molecules by including or excluding various exons or their parts in the final mRNA.
AS evolved to increase the biodiversity of proteins produced by the same single gene, and, accordingly, it adds a significant layer of complexity to the genomic coding potential. AS is highly active during development and is relevant to determining tissue-specific gene expression.
According to Ferrucci, alternative splicing is an essential component of biology that plays an important role in responses to such stressors as scarcity of energy (such as caloric restriction) or cellular senescence. Ferrucci’s group is also beginning to unravel the effect of exercise on alternative splicing, but, he admitted, “we are only scratching the surface”. It looks however that physical activity downregulates alternative splicing, which might be one of the mechanisms behind PA’s health benefits.
Lipofuscin and macular degeneration
In his talk aptly titled “Tales of the molecular garbageman”, Kelsey Moody of Ichor Life Sciences turned the audience’s attention towards another rarely mentioned player in age-related processes: lipofuscin.
Lipofuscin is a yellow-brownish, lipid-rich pigment granule which accumulates in the cells of many tissues over time, especially in post-mitotic cells such as neurons and cardiac muscle cells. Due to the age-related nature of its accumulation, lipofuscin is sometimes referred to as an “aging pigment.”
Lipofuscin granules are composed of a mix of lipids, metals (like iron), and proteins: a result of the incomplete breakdown of damaged cellular structures, mostly in the lysosomes.
While the exact implications of lipofuscin accumulation are still a topic of research, it’s generally believed to be detrimental to cells. In neurons, for instance, it can affect cellular communication and might play a role in neurodegenerative diseases.
In the retina, the accumulation of lipofuscin is associated with age-related macular degeneration (AMD), a leading cause of vision loss in older adults and a popular target of candidate geroprotective interventions. Inspired by research from SENS Foundation, Lysoclear, a company from Ichor’s portfolio, uses recombinant manganese peroxidase to counter lipofuscin accumulation in the context of AMD and has achieved some success both in vitro and in vivo. The company chose lipid nanoparticles (LNPs) as the delivery system, and the goal for IND-enabling studies has been set to spring 2024.
Neural stem cells and lipids
Anne Brunet from Stanford University talked about brain aging and rejuvenation. What makes this topic especially important is the brain’s irreplaceability. While we can hope to eventually be able to replace virtually every organ in the human body, this does not include the brain, for obvious reasons. This means that we have to find ways to rejuvenate it.
The brain has some regenerative capacity due to several of its regions harboring stem cells. However, like in other organs, brain stem cells lose function due to age-related causes, such as transcriptomic changes and a decline in proteostasis. This leads to decreased migration of those cells to other brain regions, where they could have given rise to much-needed young glial cells and neurons.
Brunet’s group focuses on lipidomic analysis of neural stem cells. According to Brunet, lipids, of which 50,000 to 150,000 varieties exist, are “vastly understudied”, and “little is known about their function, especially in the context of aging.”
Most lipids that change with age in quiescent neural stem cells are complex membrane lipids. Interestingly, neural stem cell aging is accompanied by accumulation of PUFAs (polyunsaturated fatty acids, generally considered “good fat”).
Changing lipid composition leads to less rigid and more permeable cell membranes and probably causes loss of stem cell function, but direct lipid supplementation has shown some promise against it.
Fixing the matrix
Sara Wickström from the Max Planck Institute for Molecular Biomedicine delivered a talk on another non-canonical subject: the role of the extracellular matrix (ECM) in stem cell aging. Stem cell niches are complex environments that actively affect stem cell health. As the ECM gets stiffer with age, it changes the niche’s mechanical properties. Those properties are critical for stem cell activation, as evidenced by in vitro experiments using hydrogels with variable stiffness. Scientists are not sure about how this happens, but one proposed mechanism is heterochromatin remodeling.
Bottom line: old cells experience increased mechanical stress and remain in the quiescent state for much longer, which impairs tissue regeneration. Interestingly, old cells in young mice perform normally, thus lending additional support to this hypothesis.
Another important talk on this topic was delivered by Collin Ewald from ETH Zurich, Switzerland. He talked about how a faulty ECM contributes to numerous age-related diseases. 333 ECM genes have been linked to the human “diseasesome”. In addition to this “matrisome”, Collin proposed the concept of the “matreotype” – a snapshot of ECM composition associated with or caused by a phenotype or a physiological state (such as health, disease, aging, or longevity).
Restoring the healthy remodeling of collagen, the most abundant protein in our body, might be one of the most important goals for geroscience. However, ECM-related therapies are currently at the bottom of the geroscience’s agenda, with only 8 targets and 27 clinical interventions being studied, mostly in the context of cancer fibrosis.
Collin also reported on a small-scale pilot clinical trial of DracoBelle, an extract from Moldavian dragonheads that boosts collagen production. Two-month supplementation led to marked increases in skin moisturization, elasticity, and density.
David Sinclair takes the stage
The third day of the conference saw a brief appearance of geroscience’s poster child, Harvard professor David Sinclair. Along with maintaining a high-visibility public profile, Sinclair remains a heavyweight researcher. Recently, his lab put out two important papers related to what he calls “the information theory of aging”. This theory sees loss of epigenetic information as the underlying feature of aging and claims that cells retain a “backup copy” of this information that can be used for rejuvenation.
In the first study, the lab created a murine model of genomic instability via a mild increase in double-strand breaks (DSBs) in non-essential DNA loci. According to Sinclair, some elements of DSB repair, such as Sir2, also play an important role in maintaining chromatin stability; in essence, they are guardians of both the genome and the epigenome.
How this “double duty” came to be is not clear, but it leads to increased epigenetic noise when this repair mechanism becomes too strained with age. In this study, Sinclair claimed, his team had been able to show that epigenomic dysregulation drives aging even when DSB repair is faithful and no harmful mutations occur, suggesting that loss of epigenetic information rather than mutations is indeed an underlying (or even the underlying) cause of aging.
In the second, more recent, study, Sinclair and another Harvard geroscientist, Vadim Gladyshev, collaborated on partial reprogramming using small molecules. The study produced encouraging preliminary results, although much more work is needed before reprogramming with small molecules becomes as widely accepted as that with the Yamanaka factors.
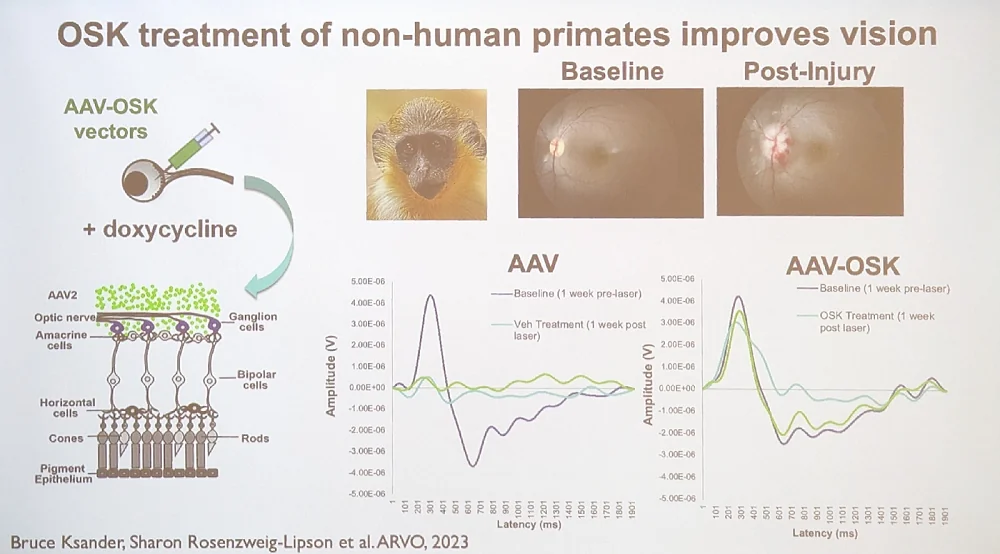
A while ago, Sinclair also made waves by restoring crushed optical nerves in mice using partial cellular reprogramming. In his talk, he reported on this year’s developments, which include a study that showed a similar improvement in vision in non-human primates.
After answering the audience’s questions in a fully packed auditorium, posing for a few selfies, and signing autographs on his popular book “Lifespan: Why We Age and Why We Don’t Have To”, Sinclair was rushed to the speakers’ dinner, ending this rare in-person appearance.
Aging trajectories, senescence, and cancer
One of the conference’s organizers, Prof. Morten Scheibye-Knudsen, reported on his team’s work, which includes trying to understand various trajectories of aging using unconventional data such as medical records. These records, according to Scheibye-Knudsen, strongly correlate with age and reveal aging’s complexity and variability. For instance, aging is gender-specific, with males and females tending to have different trajectories (“males age faster but later, and females earlier but slower”, as Scheibye-Knudsen put it). Of course, aging trajectories also differ on an individual level.
Organs and tissues also age in different ways: for example, the liver, according to Scheibye-Knudsen, ages in an almost linear fashion, while in the lung, aging progresses gradually (that is, steadily but not necessarily linearly). It appears that it is possible to predict age from a tissue’s (specifically, lung tissue) clinical features.
Scheibye-Knudsen also talked about his team’s project that involves gleaning insights into possible anti-aging interventions by associating terms in scientific papers’ abstracts to aging. As an example, he brought up nintedanib, which showed a senolytic effect in fruit flies.
Scheibye-Knudsen had more news on cellular senescence (a popular topic at the conference). In a paper currently published as a pre-print, his team shows a link between cellular senescence and risk of cancer. Senescence is known to have a complex relationship with cancer: it can be both a barrier to cancer and its promoter, depending on the context. By analyzing senescence-associated cellular morphological features in about 4000 non-malignant biopsies the team was able to predict the risk of developing breast cancer in the future.
At the end of his talk, Scheibye-Knudsen announced the founding of the Nordic Aging Society – yet another organization that aims to help aging research and advocacy.
Fasting without fasting
Prof. Valter Longo of USC Davis School of Gerontology, a prominent geroscientist who rarely appears at conferences, talked about the fasting-mimicking diet (FMD), multi-system regeneration, and longevity.
FMD was originally developed for cancer patients to recapitulate the beneficial effects of water fasting, which is hard to maintain. It differs from keto diets in several aspects, including low protein consumption and a less strict position on carbohydrates. Unlike keto, FMD is a periodic diet, not intended to be followed continuously.
According to Longo, FMD leads to life extension and rejuvenation of the immune system in mice. It also appears to be beneficial for stem cell function by boosting proliferation and differentiation, including into insulin-producing ß-cells. In 2017, a study showed that FMD reverses hyperglycemia and prevents death in a mouse model of type 2 diabetes. FMD cycles can also reverse type 1 diabetes in a mouse model.
What about human trials? Longo presented some promising unpublished data from a very recent one, but we are not at liberty to divulge it yet.
Mitochondria: central to both apoptosis and senescence
Among the several speakers who talked about cellular senescence was João Passos of Mayo Clinic. In his talk, Passos raised the painful topic of senescence heterogeneity, which hampers research in this otherwise promising area. While several senescence markers have been used in studies, not all of them are present in every senescent cell. For instance, both p16 and p21 kinase inhibitors are considered markers of senescence, but Passos showed that they have very different dynamics and can be expressed transiently. The senescence-associated secretory phenotype (SASP), can also differ considerably between senescent cell subtypes.
ß-galactosidase, another popular senescence marker, can be present in non-senescent cells, such as activated macrophages. Inflammatory factors commonly associated with SASP can be produced by non-senescent cells too. Passos concluded that senescent cells should be identified via multi-marker approaches, including by spatial methods with single cell resolution, since senescent cells can be quite rare and far apart. “Single cell transcriptomics, proteomics, epigenomics will likely be the optimal way to detect senescence and its heterogeneity”, he said.
The second part of the talk was devoted to why we need to understand mitochondria in order to develop senotherapies. Mitochondrial dysfunction, Passos said, is an often-unappreciated hallmark of senescence. Mitochondria are actually required for SASP production. A major mechanism behind this link is that mitochondrial DNA leaking into the cytoplasm through the membrane of dysfunctional mitochondria is recognized by the cytosolic-DNA sensing cGAS–STING pathway. This activates pro-inflammatory genes and SASP production, which can be prevented by clearing out diseased mitochondria.
But why do dysfunctional mitochondria leak mtDNA? Apparently, it happens due to MOMP (mitochondrial outer membrane permeability) mediated by the proteins BAK and BAX. Originally, this is supposed to trigger apoptosis, meaning both apoptosis and senescence are regulated by similar mitochondria-related processes. Inhibition of the BAK/BAX complex suppresses mtDNA leakage and SASP production and improves healthspan in mice. Interestingly, stopping apoptosis is essentially the opposite of what senolytics are trying to do. According to Passos, it might be wise to go after the SASP while keeping damaged cells arrested instead of trying to nudge them towards apoptosis.
The 106th talk
Somewhat symbolically, in a closing talk or the conference named “Final Line, Final Talk: Defying Time, Defying the Clock”, Harvard Prof. Vadim Gladyshev went back to basics: namely, to the fundamental question of what aging is. He reminded the audience that among the 106 talks at the conference, very few were about aging per se. Is aging the continuous accumulation of damage or functional decline, or should we measure it via disease burden or mortality?
Gladyshev’s view is that aging starts with damage, while almost the entire field of longevity biotechnology is currently focusing on late manifestations of aging. But how do we design experiments that measure aging? Gladyshev suggested that in order to target aging, scientists must identify multi-marker “signatures” of longevity (those associated with increased lifespan, including across species), of aging (those associated with processes of aging), and of rejuvenation (those associated with the rare events of transitioning from an ‘older’ to a ‘younger’ phenotype).
Gladyshev is fascinated with the rejuvenation event (sometimes called “the embryonic reset”) that occurs during early development and allows an old organism to produce perfectly young offspring. Like many other geroscientists, Gladyshev thinks this event might hold the keys to understanding and eventually defeating aging. He announced a new paper from his lab on this topic, currently in press with Aging Cell. We will make sure to cover this paper as soon as it is published.




