A study published in Cell Reports has shown how the activation of a common protein in neurons improves memory in both worms and mammals.
Common between animals
This paper begins with a striking fact: C.elegans worms, mice, and people all lose memory with age [1] and for at least one of the same reasons: the cAMP element binding protein (CREB), which is a necessary part of the neural plasticity that allows the formation of new long-term memories [2], declines with age [3].
In worms, EGL-30 activates CREB to stimulate memory formation [4] and has been found to be critical to their behavior. In mammals, GNAQ appears to do the same thing [5], although it is less well-studied. This paper, therefore, takes a look at these compounds’ effects on CREB in both worms and mice and how it can affect memory..
Restoring memory until the end of life
Normally, C.elegans worms lose their ability to form long-term memories at four days old [6], as measured by teaching them food-smell associations. While these researchers had previously found that activating a gain-of-function EGL-30 mutation restored memory in these worms [7], they did not know how late in life this could be applied. Therefore, they used genetically modified worms that express EGL-30 as a heat shock protein and activated it at day 6 of the worms’ lifespans.
The worms gained far more ability to associate food and smells, remembering things on day 7 that they had learned on day 6 with far more ability than the control group. Howeve, past day 8, the effects can no longer be measured: the worms lose their ability to move before they lose their memory. Gain-of-function EGL-30 activation appears to only affect memory, as it did nothing to rescue the worms’ motor function, nor did it improve lifespan at all.
The mammalian equivalent restores mouse memory
These researchers confirmed previous work showing that GNAQ declines with normal aging [8]. Specifically, they focused on the hippocampus, the part of the brain in which new memories are formed. They found that every part of the murine hippocampus had decreased GNAQ expression.
The researchers then compared three groups of mouse neurons. One had increased wild-type GNAQ, another had a gain-of-function mutation similar to the worms they had used, and there was a control group. Only the neurons in the gain-of-function group had increased in size and complexity. The gene expression of this group was also greatly altered compared to either of the other groups.
This mutation was then tested in very old mice. 24-month-old mice were given lentiviral vectors that caused them to express it. As expected, these mice had increased expression of CREB and its downstream factors, demonstrating the modified GNAQ’s biological effects. Downstream genes related to synaptic plasticity, and the complexity and length of synapses, were improved just as they were in the cellular study.
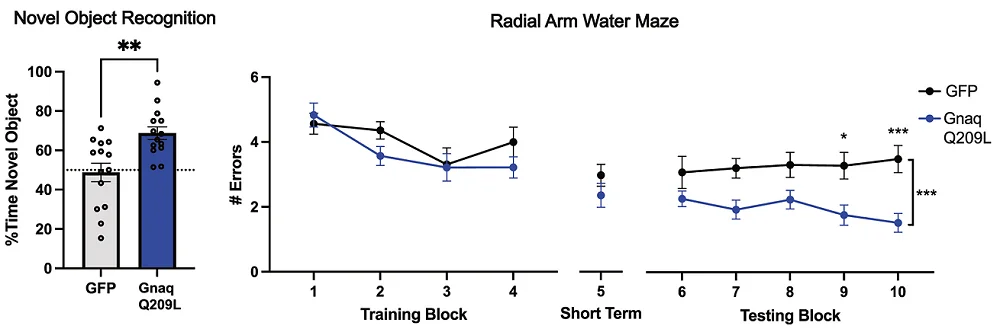
Behaviorally, the mice, like the worms, had very specific improvements. While short-term memory was unaffected, treated mice nested more like younger animals, and their long-term memory was significantly improved, as shown by remarkably fewer errors on a water maze test.
A specific and powerful effect
These researchers note many of the things that the gain-of-function GNAQ mutation does not do. It doesn’t improve neurogenesis, the formation of new neurons, and it doesn’t seem to have any effects on inflammation or microglia. Therefore, the researchers suggest that other memory-improving interventions may work alongside a GNAQ-based intervention for a greater total effect. They also suggest that GNAQ-based interventions may improve memory in younger people as well, although this was not tested in mammals.
Literature
[1] Arey, R. N., & Murphy, C. T. (2017). Conserved regulators of cognitive aging: From worms to humans. Behavioural brain research, 322, 299-310.
[2] Lakhina, V., Arey, R. N., Kaletsky, R., Kauffman, A., Stein, G., Keyes, W., … & Murphy, C. T. (2015). Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron, 85(2), 330-345.
[3] Villeda, S. A., Plambeck, K. E., Middeldorp, J., Castellano, J. M., Mosher, K. I., Luo, J., … & Wyss-Coray, T. (2014). Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature medicine, 20(6), 659-663.
[4] Brundage, L., Avery, L., Katz, A., Kim, U. J., Mendel, J. E., Sternberg, P. W., & Simon, M. I. (1996). Mutations in a C. elegans Gqa gene disrupt movement, egg laying, and viability. Neuron, 16(5), 999-1009.
[5] Qian, N. X., Winitz, S., & Johnson, G. L. (1993). Epitope-tagged Gq alpha subunits: expression of GTPase-deficient alpha subunits persistently stimulates phosphatidylinositol-specific phospholipase C but not mitogen-activated protein kinase activity regulated by the M1 muscarinic acetylcholine receptor. Proceedings of the National Academy of Sciences, 90(9), 4077-4081.
[6] Kauffman, A. L., Ashraf, J. M., Corces-Zimmerman, M. R., Landis, J. N., & Murphy, C. T. (2010). Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS biology, 8(5), e1000372.
[7] Arey, R. N., Stein, G. M., Kaletsky, R., Kauffman, A., & Murphy, C. T. (2018). Activation of Gaq signaling enhances memory consolidation and slows cognitive decline. Neuron, 98(3), 562-574.
[8] Peng, S., Zeng, L., Haure-Mirande, J. V., Wang, M., Huffman, D. M., Haroutunian, V., … & Tu, Z. (2021). Transcriptomic changes highly similar to Alzheimer’s disease are observed in a subpopulation of individuals during normal brain aging. Frontiers in Aging Neuroscience, 13, 711524.