A recent publication in Aging and Disease describes human and animal research demonstrating improvement in the function and biomarkers of muscle tissue after Ishige okamurae extract supplementation [1].
The benefits of seaweed
Clinical trials of seaweed consumption have reported beneficial health effects and protection against senescence-associated and metabolic diseases, such as cardiovascular disease, cancer, obesity, and diabetes mellitus [2,3].
Other studies are testing the impact of seaweed consumption on various age-related conditions. This particular study tested the impact of seaweed consumption on sarcopenia, a condition in which patients gradually lose muscle mass and strength. Such loss restricts patients’ mobility, increases frailty, and lowers their quality of life [4].
A cohort study performed on middle-aged and elderly Korean populations has suggested that a seafood and seaweed diet can help in sarcopenia prevention [5]. Preclinical animal research also suggests improvements in muscle functions following seaweed consumption, specifically Ishige okamurae.
Another study investigating I. okamurae extract (IO) and DPHC (a marine polyphenol from I. okamurae) showed that both of them “were able to reverse the aging parameters and sex hormonal imbalance in 14-month-old female C57BL/6J mice” [6].
Animal and human research show promise
In this study, the researchers followed 64 participants between 50 to 85 years. Participants received IO supplements or placebo for 12 weeks. The researchers assessed the difference between leg muscle strength at baseline and after 12 weeks of treatment. Leg strength was used as a marker because it is known to correlate with senescence progression [7].
As clinical biomarkers remained within normal values, IO was reported to be safe for the participants. The muscle testing revealed that “daily intake of IO helps prevent the decrease in muscle strength due to aging.”
To gain more understanding on the molecular level, the researchers turned to 12-month-old male mouse models. These mice received IO daily for 6 weeks.
The researchers started by testing their testosterone levels, as testosterone is known to promote muscle growth and protein synthesis [8]. Previous research in female mice has reported that IO alleviates age-related sexual hormone deficiencies [6].
IO supplementation improved testosterone serum levels and androgen receptor expression in the gastrocnemius muscle tissue compared to controls. In the control group, androgen receptor expression declined, but in the supplemented group, testosterone and androgen receptor levels were physiologically normal.
Most importantly, IO supplementation positively impacted the muscle mass of the treated mice. While bone mineral density and fat mass didn’t significantly differ between the placebo and IO-supplemented groups, the researchers noted that in the IO-supplemented group, mice had significantly increased lean mass compared to the age-matched placebo group. This increase was dose-dependent.
Muscle strength deteriorates as mice age. However, 6-week IO supplementation led to an increase in grip strength:
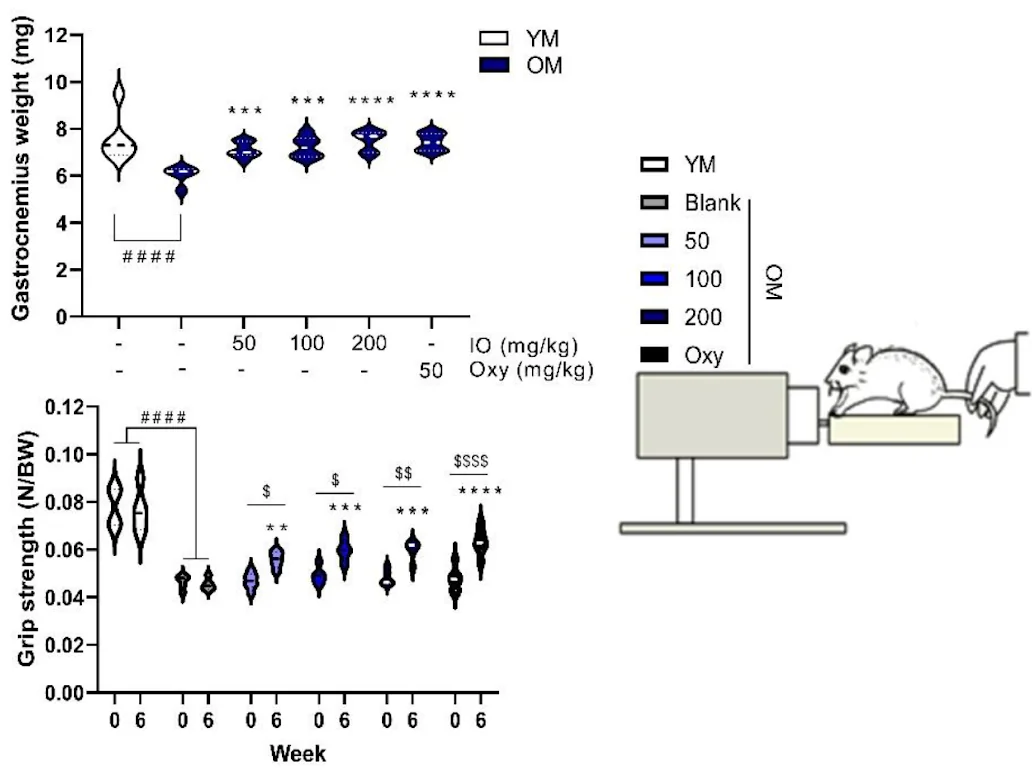
This increase in muscle growth and inhibition of muscle decomposition was also reflected in protein levels. IO treatment was found to downregulate expression of Atrogin-1, the protein implicated in protein degradation, and it significantly enhanced expression of myogenin, the primary myogenesis inducer [9,10].
IO supplementation also improved different markers of muscle health. One of them, Pax-7+, indicates “potential recovery ability against muscle damage” [11]. As expected, Pax-7+ levels were reduced in aged, control-fed mice compared to young ones. IO supplementation led to significant increases in Pax-7+ expression in a dose-dependent manner.
The proper functioning of muscles relies heavily on their mitochondria since they produce ATP, a molecule that serves as an energy source [12]. The authors used mitochondrial biogenesis processes, reflected by mitochondria complex protein expression, as a biomarker to evaluate muscle health. In general, mitochondrial complexes are sensitive to aging and are decreased in older mice compared to young ones. However, IO supplementation led to an increase of mitochondria complex protein expression.
Lack of mechanical understanding
As of now, there is no effective way to stop or reverse sarcopenia. While exercise can help in delaying the age-related loss of muscle mass and function, elderly people generally have limited ability to exercise. That makes the need for other ways to alleviate muscle loss even more pressing.
The authors propose seaweed consumption as an easy-to-implement possible remedy. However, as of now, there is no established protocol or dosing or any other recommendations regarding such an intervention. The authors also recommend establishing additional safety evidence, and they stress the need to better understand the molecular pathways linking seaweed consumption to muscle functioning.
Regular intake of the brown alga I. okamurae can prevent age-related muscle weakness. A consistent seaweed diet may assist in the prevention of age-related diseases. However, large-scale studies are required to further elucidate the protective function of seaweeds against sarcopenia caused by aging.
Literature
[1] Hyun, J., Lee, S. Y., Ryu, B., & Jeon, Y. J. (2023). A Combination Study of Pre- and Clinical Trial: Seaweed Consumption Reduces Aging-Associated Muscle Loss!. Aging and disease, 10.14336/AD.2023.0927. Advance online publication.
[2] Iso, H., Kubota, Y., & Japan Collaborative Cohort Study for Evaluation of Cancer (2007). Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pacific journal of cancer prevention : APJCP, 8 Suppl, 35–80.
[3] Murai, U., Yamagishi, K., Sata, M., Kokubo, Y., Saito, I., Yatsuya, H., Ishihara, J., Inoue, M., Sawada, N., Iso, H., Tsugane, S., & JPHC Study Group (2019). Seaweed intake and risk of cardiovascular disease: the Japan Public Health Center-based Prospective (JPHC) Study. The American journal of clinical nutrition, 110(6), 1449–1455.
[4] Cao, L., & Morley, J. E. (2016). Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. Journal of the American Medical Directors Association, 17(8), 675–677.
[5] Kim, S. A., Ha, J., Lim, B., Kim, J. M., & Shin, S. (2020). The Association between Major Dietary Pattern and Low Muscle Mass in Korean Middle-Aged and Elderly Populations: Based on the Korea National Health and Nutrition Examination Survey. Nutrients, 12(11), 3543.
[6] Hyun, J., Ryu, B., Oh, S., Chung, D. M., Seo, M., Park, S. J., Byun, K., & Jeon, Y. J. (2022). Reversibility of sarcopenia by Ishige okamurae and its active derivative diphloroethohydroxycarmalol in female aging mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 152, 113210.
[7] Marcell, T. J., Hawkins, S. A., & Wiswell, R. A. (2014). Leg strength declines with advancing age despite habitual endurance exercise in active older adults. Journal of strength and conditioning research, 28(2), 504–513.
[8] Vingren, J. L., Kraemer, W. J., Ratamess, N. A., Anderson, J. M., Volek, J. S., & Maresh, C. M. (2010). Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports medicine (Auckland, N.Z.), 40(12), 1037–1053.
[9] Bentzinger, C. F., Wang, Y. X., & Rudnicki, M. A. (2012). Building muscle: molecular regulation of myogenesis. Cold Spring Harbor perspectives in biology, 4(2), a008342.
[10] Gomes, M. D., Lecker, S. H., Jagoe, R. T., Navon, A., & Goldberg, A. L. (2001). Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proceedings of the National Academy of Sciences of the United States of America, 98(25), 14440–14445.
[11] Chen, W., Datzkiw, D., & Rudnicki, M. A. (2020). Satellite cells in ageing: use it or lose it. Open biology, 10(5), 200048.
[12] Marzetti, E., Calvani, R., Cesari, M., Buford, T. W., Lorenzi, M., Behnke, B. J., & Leeuwenburgh, C. (2013). Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. The international journal of biochemistry & cell biology, 45(10), 2288–2301.




