A new study suggests that the dysregulation of the crosstalk between hypothalamus and white adipose tissue affects aging. Rescuing this “communication channel” led to significant lifespan extension [1].
It’s all in your brain
Thinking is not the brain’s sole responsibility. Some brain regions, such as the hypothalamus, control a plethora of bodily processes that don’t involve any conscious thoughts. This constantly humming communication between the brain and various organs and tissues gets less efficient as we age, contributing to various other aspects of aging [2].
In this new study published in Cell Metabolism, the researchers describe a subset of neurons that reside in dorsomedial hypothalamus (DMH) and are characterized by the expression of the protein Ppp1r17, and their surprising effect on aging in mice.
When fat gets lazy
The researchers suspected that these neurons communicate with a specific tissue, but they didn’t know which one. To elucidate this, they knocked down Ppp1r17 in young mice via RNA interference in a DMH-specific manner. Over the period of two months after the treatment, those genetically modified mice gained a lot of weight, all of which came from fat. Their food intake increased, even as their activity levels went down significantly. Ablation of Ppp1r17-positive DMH neurons led to similar results.
Characteristics of WAT were altered in the genetically modified mice; adipocytes (fat cells) were larger and contained more fat than in normal mice. Further analysis showed decreased WAT innervation, suggesting that Ppp1r17-positive DMH neurons directly regulate WAT function via the sympathetic nervous system.
Interestingly, WAT in modified mice also had lower levels of NAMPT, a rate-limiting enzyme central to biosynthesis of another molecule, NAD+, which mediates energy production. NAD+ also serves as a substrate for a family of longevity-related enzymes called sirtuins. Some of these enzymes, such as SIRT1, have been linked to brain health [3].
Improved health and lifespan
Differential gene expression analysis found that Ppp1r17 regulates neurons’ synaptic function. It seems that, normally, Ppp1r17 is mostly located in the cell’s nucleus, where it regulates transcription of other genes, but with age, more of it is translocated from the nucleus to the cytoplasm. A protein, PKG, is the driver of this process.
When PKG expression was knocked down specifically in DMH neurons of 21-month-old mice, they exhibited attenuation of frailty and increased activity compared to age-matched controls. Moreover, the researchers detected a significant 7% extension in mean lifespan as well as a borderline significant increase in maximum lifespan. The treated mice also had higher levels of extracellular NAMPT (eNAMPT), which has been shown to improve hypothalamic function [4].
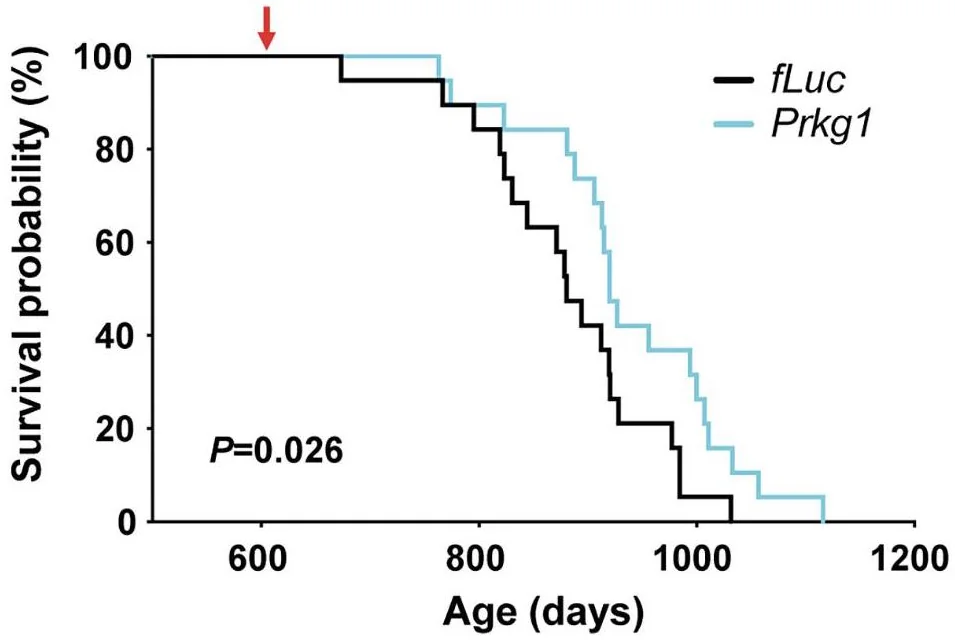
Slower rate of aging
The researchers then tried a different approach: chemical activation of Ppp1r17-positive DMH neurons. Increasing these neurons’ activity led to results similar to those of PKG knockdown: improved health and increased lifespan. Interestingly, the treated mice also exhibited a significant delay in age-related mortality at all ages. This hints at “true rejuvenation” (slower rate of aging across the lifespan) as opposed to “compression of mortality”, which we often see in anti-aging studies and describes a situation in which animals stay healthier for longer but then experience accelerated decline. In that situation, there is no meaningful extension of maximum lifespan.
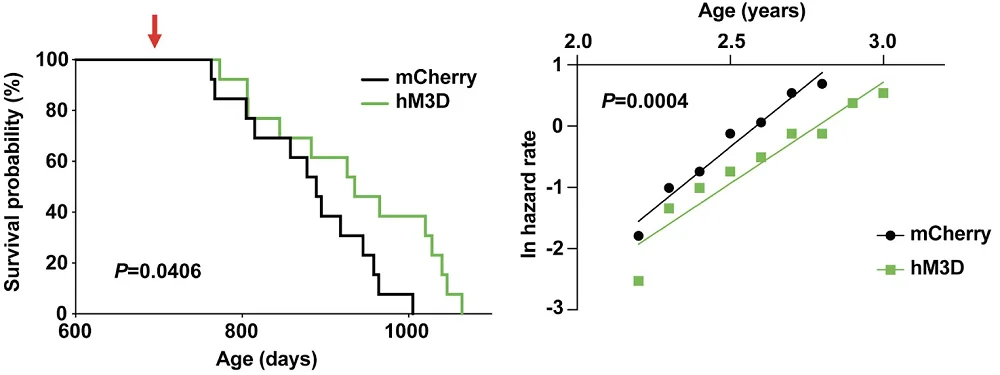
In summary, this study uncovered an intriguing interplay between the brain and white adipose tissue, which help each other perform their respective tasks until aging disrupts the communication between them. There are several possible routes from here to anti-aging interventions, and one of them involves NAMPT supplementation. “We can envision a possible anti-aging therapy that involves delivering eNAMPT in various ways,” said the study’s senior author, Shin-ichiro Imai, MD, PhD, from Washington University School of Medicine in St. Louis. “We already have shown that administering eNAMPT in extracellular vesicles increases cellular energy levels in the hypothalamus and extends life span in mice.”
Our study demonstrates the importance of DMHPpp1r17 neurons, a key neuronal subpopulation in the cDMH, in controlling the process of aging and determining lifespan in mice. Age-associated decline in DMHPpp1r17 neuronal activity leads to age-associated pathophysiological changes, including significant reduction in physical activity, lipolysis, and eNAMPT secretion from WAT. Genetic and chemogenetic activation of DMHPpp1r17 neurons in aged mice mitigates age-associated physiological decline and extends lifespan. These findings provide critical insights into the systemic regulatory network for mammalian aging and longevity control and the development of more effective anti-aging interventions.
Literature
[1] Tokizane, K., Brace, C. S., & Imai, S. I. (2024). DMHPpp1r17 neurons regulate aging and lifespan in mice through hypothalamic-adipose inter-tissue communication. Cell Metabolism.
[2] Imai, S. I. (2016). The NAD World 2.0: the importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control. NPJ systems biology and applications, 2(1), 1-9.
[3] Ng, F., Wijaya, L., & Tang, B. L. (2015). SIRT1 in the brain—connections with aging-associated disorders and lifespan. Frontiers in cellular neuroscience, 9, 64.
[4] Yoon, M. J., Yoshida, M., Johnson, S., Takikawa, A., Usui, I., Tobe, K., … & Imai, S. I. (2015). SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell metabolism, 21(5), 706-717.