Red Blood Cells as Biomarkers of Aging
The advent of artificial intelligence and its application to the study of aging and longevity took a giant leap forward in 2013 with the development of tools for measuring biological age. Public access to these tools opened a fascinating opportunity for self-studies into how diet, lifestyle, exercise, and supplementation habits could affect someone’s rate of aging or likelihood of death over time.
Artificial intelligence and the measurement of biological age
In 2013, Steve Horvath developed a highly accurate artificial intelligence-driven method of determining biological age [1]. This long-awaited development ushered in a new era of aging research. For the first time, it enabled researchers in academia and industry to directly measure some of the results of their work without waiting for lifespans to end.
For example, in 2019, Dr. Greg Fahy conducted an experiment aimed at thymus gland regeneration. There were nine participants in the year-long study. In addition to partial regeneration of the participants’ thymus glands, their average biological age based on DNA methylation patterns decreased by 2.5 years [2].
Since then, researchers have developed many AI-based methods for assessing biological age and mortality. Mortality is defined as the rate of death in a given population during a specified interval [3]. A brief glance at aging-related studies at clinicaltrials.gov reveals the wide variety of interventions currently under study.
Public access and self-study
Researchers have made these biological age assessment tools accessible to the public, giving individuals the opportunity to self-experiment with diet, lifestyle, exercise, supplementation, and other measures [4].
One such avenue of biological age testing that is low cost, accessible, and accurate is provided by Levine’s Phenotypic Age Calculator and Aging.ai’s 1.0, 2.0, and 3.0 assays. These estimators simply require the user to enter standardized blood test values into an online platform and hit the send button. Some insurance companies cover this blood testing.
The need for biomarkers
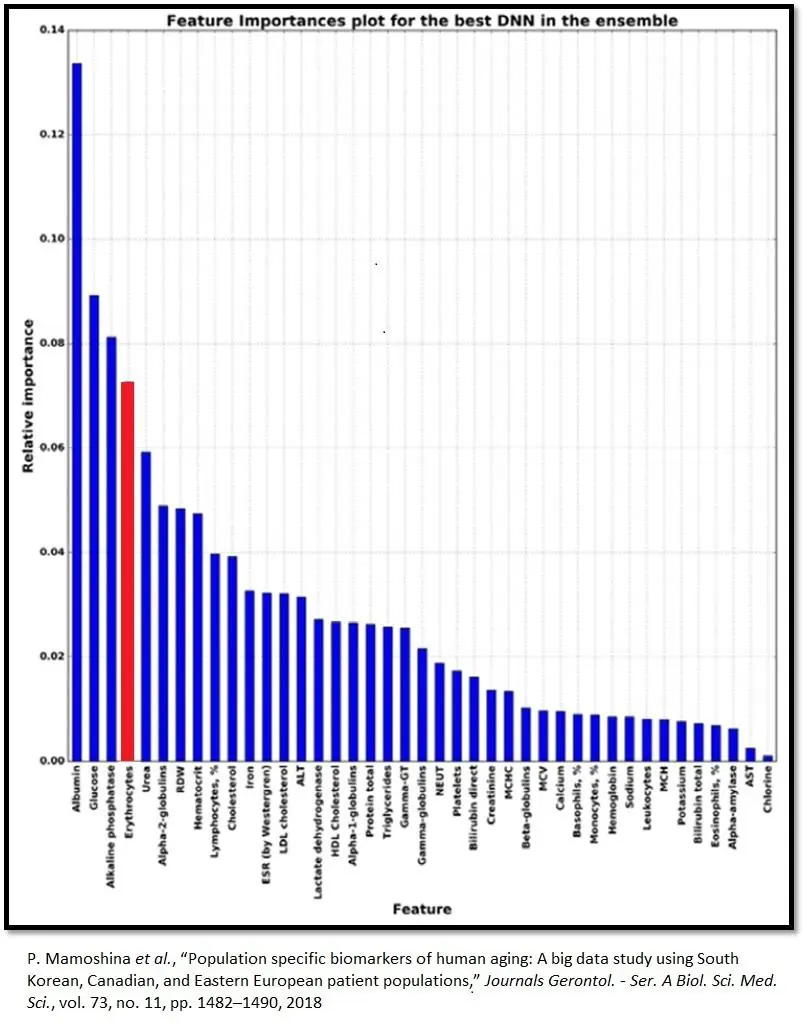
Biomarker values provides a starting point that yields insights into individual physiology and how lifestyle changes, tracked over time, impact it. Red blood cells (erythrocytes) are of particular interest here, as they rank fourth in relative importance among Aging.ai’s big five blood biomarkers. The other four are albumin, alkaline phosphatase, glucose, and urea.
Red blood cell counts can reflect many facets of human health
Erythrocytes transport oxygen through the body to cells and return carbon dioxide back to the lungs for removal from the body [5]. Red blood cell counts can reflect deficits in many different body systems [6].
The presence of red blood cells decreases with age [7], and the quality and quantity of red blood cells can be indicative of systemic failures associated with multiple aging processes. This may account for why red blood cell counts are so good at predicting biological age.
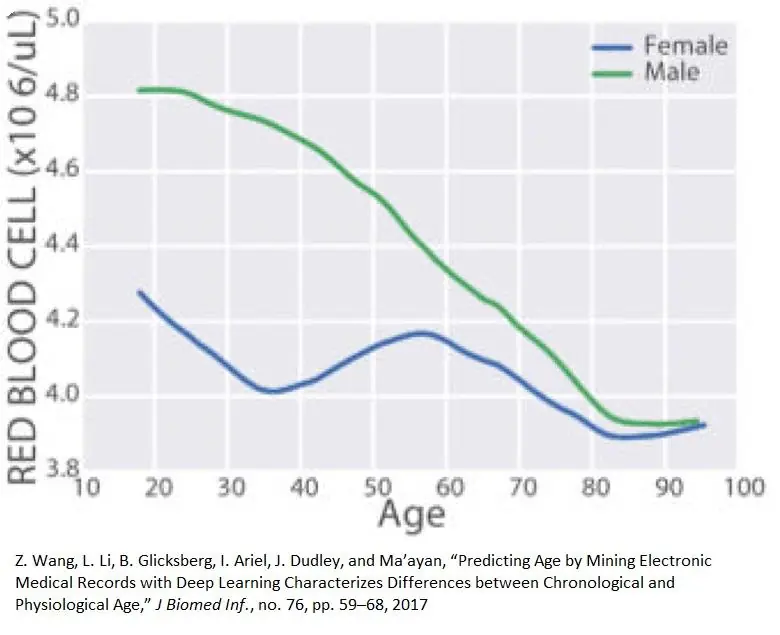
Red blood cell generation (erythropoiesis)
Age-related decreases in the quantity and quality of red blood cells occur because of changes in the rate of red blood cell production. Erythropoiesis is the process of making red blood cells [8]. Erythropoietin (EPO) is a hormone produced in the interstitial cells of the kidney and released to trigger erythropoiesis through its attachment to the EpoR receptor on burst-forming unit-erythroid cells in bone marrow. Upon EPO’s attachment to the EpoR receptor, the cell undergoes a series of transformations, which end in the formation of red blood cells [9].
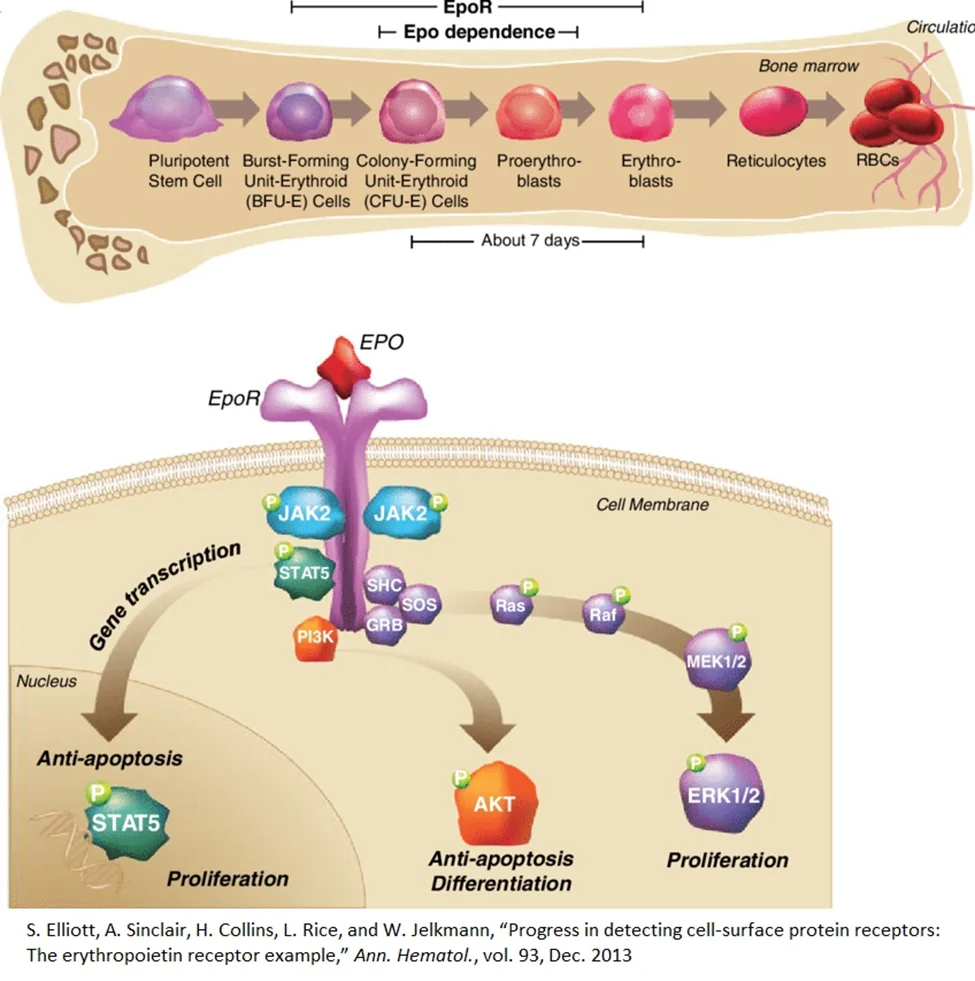
Red blood cell production is proportional to EPO production, and the production of EPO influences many body systems. EPO is a multifunctional protein released by the kidneys under conditions of hypoxia (low oxygen concentrations) [6]. Hypoxia inducible factors (HIFs) control this process within the kidney by changing form at different oxygen concentrations [10].
When oxygen concentration is normal, the ubiquitin proteosome system quickly marks HIFs for destruction and degrades them. However, when oxygen concentrations are low, the ubiquitin proteosome system does not mark HIFs for destruction, and they translocate to the nucleus of kidney cells to activate the gene for EPO, which will then enter circulation and make its way to bone marrow to prompt red blood cell production [11].
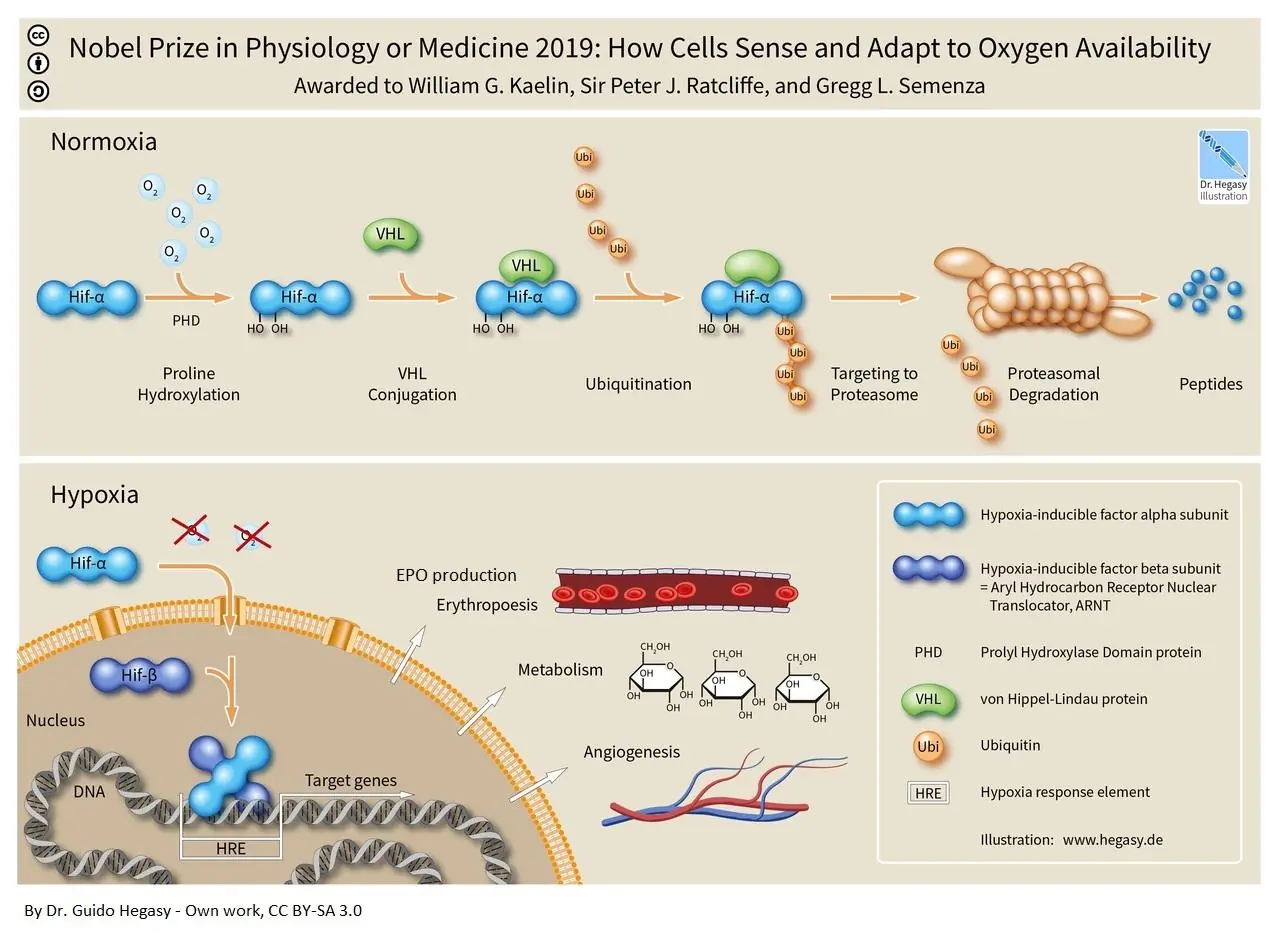
However, shortfalls in the production of EPO not only underwrite red blood cell production, they also impact many other tissues, including those in the nervous system, cardiovascular system, musculoskeletal system, and immune system. In addition, EPO affects metabolism in a variety of ways.
EPO plays a critical role in neuroprotection and repair of nerve tissue. EPO and its receptor are expressed in the nervous system, and EPO provides neuroprotection in cell culture and animal models of nervous system disorders [12].
EPO effects nitric oxide metabolism and thereby regulates tension in the blood vessel walls (vascular endothelium) [13]. EPO provides protection of the heart muscle when it is deprived of oxygen [14] and helps in the maintenance and repair of skeletal muscle [15].
Additionally, EPO helps prevent inflammation from accumulation of fat in male mice during diet-induced obesity and helps prevent diet-induced obesity [16]. Lastly, EPO helps maintain normal bone development and remodeling [17].
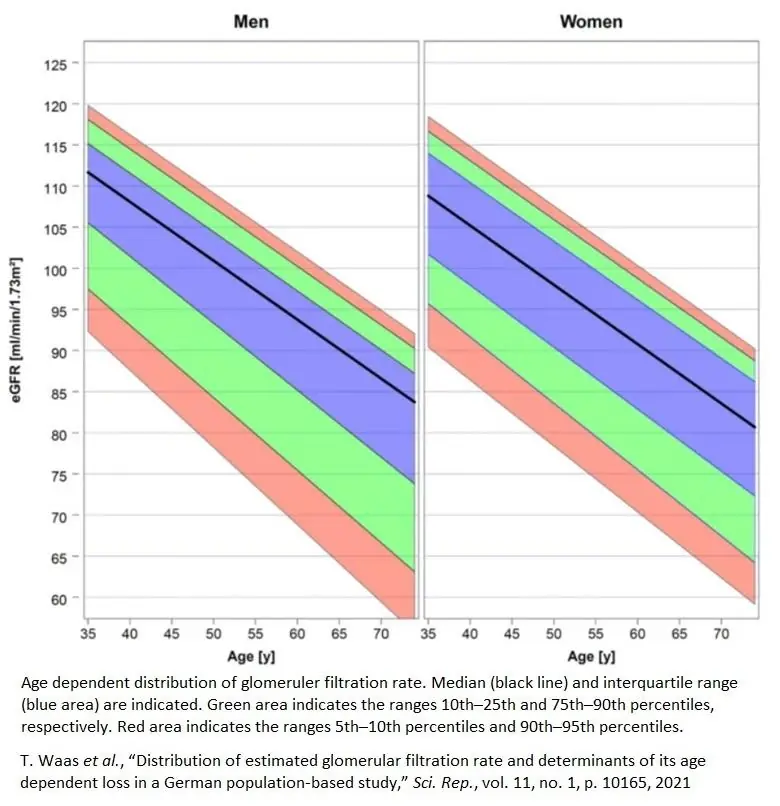
Kidney function, as measured by glomerular filtration rate and other metrics, declines linearly with age beginning at about 35 [18].
EPO and aging
EPO can increase or decrease with age, but it generally increases. Interestingly, individuals with diabetes frequently have an impaired ability to produce EPO, and this is likely secondary to diabetes-induced kidney damage [19,20].
Researchers believe that non-diabetics experience increased EPO levels due to a variety of causes, including compensation for subclinical blood loss, increased red blood cell turnover, or increased erythropoietin resistance of red cell precursors [21].
The hematopoietic stem cell niche
Thus far we have covered a range of factors that act on hematopoietic stem cells in bone marrow to drive the differentiation and proliferation of these cells to their final fate- red blood cells. However, as the hematopoietic stem cell niche (the pool of stem cells from which red blood cells arise) ages. Hematopoietic stem cells (HSCs) ensure a balanced production of all blood cells throughout life. As they age, HSCs gradually lose their self-renewal and regenerative potential. This suggests that even if most of the right factors are in place- sufficient iron, folate, B12, and healthy kidneys that produce ample amounts of EPO- red blood cell counts can still drop due to a failing response on the part of hematopoietic stem cells [22].
It is believed that most of the causes that underpin HSC aging are a result of inherent changes that occur in stem cells that are not a function of the microenvironment in which the stem cells reside. Many hematological pathologies are strongly age-associated, and strategies to intervene in aspects of the stem cell aging process may have significant clinical relevance [22].
B6, B12, folate, and iron in erythropoiesis
Beyond EPO, several nutritional factors can contribute to low red blood cell counts including folate, vitamin B12, and Iron. Red blood cells require folate and vitamin B12 for developing red blood cells to divide and become mature red blood cells. Deficiency of folate or vitamin B12 impairs DNA synthesis and causes red blood cells to self-destruct, which results in a lack of red blood cells from ineffective erythropoiesis [23].
In a small study, researchers examined the effect of vitamin B-6 on plasma and red blood cell concentration. Scientists evaluated the effect of 100 mg of vitamin B6 twice a day on plasma and red blood cell magnesium over a period of one month. According to reported normal ranges for plasma and red blood cell magnesium (1.7 to 2.3 and 4.7 to 7.0, mg per dl, respectively), three subjects had low plasma magnesium, and all subjects had low RBC magnesium during the control period. Following vitamin B6 administration, the mean plasma and red blood cell magnesium levels were significantly elevated, with a doubling of RBC levels after four weeks of therapy. These results support the postulate that vitamin B6 plays a fundamental role in the active transport of minerals across cell membranes [24].
B12 absorption is known to deteriorate with aging . The release of B12 from food and its subsequent absorption into the body is a complex process that requires the coordination of the stomach, pancreas, and intestine. Multiple co-morbidities, combined with multiple prescription drugs, and increasing dependency associated with ageing can lead to malnutrition due to inadequate intake and malabsorption of vitamin B12, resulting in deficiency [25].
Folate deficiency is also more likely to increase with advancing age for the same reasons, and absorption of B12 is also impaired by comorbidities and use of multiple prescription drugs [26].
The most functionally relevant protein in red blood cells is hemoglobin. Hemoglobin is a protein with metal in it: a metalloprotein. In the case of hemoglobin, that metal is iron. Therefore, the production of red blood cells requires the timely delivery of iron to red blood cell precursors. Red blood cells contain two-thirds of the body’s total iron content [27].
Iron absorption, plasma iron concentrations, and tissue iron distribution are tightly controlled by the liver-produced hormone hepcidin [27]. Hepcidin not only affects red blood cell production by how well it regulates iron metabolism, it affects obesity along with the immune response, as immune cells depend on iron [28].
The dysregulation of iron trafficking over time may account to two seemingly contradictory phenomena. Iron deficiencies are common among elderly people, and iron accumulation occurs with aging and is associated with many age-related diseases. Iron accumulation is problematic because of iron’s reactive potential. Researchers have also shown that excessive iron decreases lifespan in several model organisms [29].
Many life-extending interventions, such as rapamycin, calorie restriction, and old plasma dilution can be explained by the effects they have on iron absorption, excretion, and metabolism. Control of body iron stores so that they remain in a low normal range may be an important lifespan- and healthspan-extending intervention [29-31].
Red blood cells counts tend to fall with age. Primary contributing factors are inadequate EPO production consequent to declining kidney function and poor nutrition most often associated with poor absorption of Vitamins B6, B12, folate, and iron. The declining potency of the hematopoietic stem cell niche reduces the rate of red blood cell production as well.
Kidney health and longevity can be optimized by following established dietary and exercise protocols for optimal cardiovascular health, controlling hypertension, and controlling blood glucose levels. Ideally, nutrients essential to red blood cell production should be acquired through good eating habits. In some cases, however, supplementation may be necessary.
Literature
[1] S. Horvath, “DNA methylation age of human tissues and cell types,” Genome Biol., vol. 14, no. 10, p. 3156, 2013
[2] G. M. Fahy et al., “Reversal of epigenetic aging and immunosenescent trends in humans,” Aging Cell, vol. 18, no. 6, p. e13028, Dec. 2019
[3] J. Hernandez and P. Kim, Epidemiology Morbidity And Mortality. Treasure Island (Fl): StatPearls Publishing, 2022
[4] D. Zeevi et al., “Personalized Nutrition by Prediction of Glycemic Responses,” Cell, vol. 163, no. 5, pp. 1079–1094, 2015
[5] V. Kuhn et al., “Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia,” Antioxid. Redox Signal., vol. 26, no. 13, pp. 718–742, May 2017
[6] S. Suresh, P. K. Rajvanshi, and C. T. Noguchi, “The Many Facets of Erythropoietin Physiologic and Metabolic Response,” Front. Physiol., vol. 10, no. January, pp. 1–20, 2020
[7] Z. Wang, L. Li, B. Glicksberg, I. Ariel, J. Dudley, and Ma’ayan, “Predicting Age by Mining Electronic Medical Records with Deep Learning Characterizes Differences between Chronological and Physiological Age,” J Biomed Inf., no. 76, pp. 59–68, 2017
[8] M. Moras, S. D. Lefevre, and M. A. Ostuni, “From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals ,” Frontiers in Physiology , vol. 8. 2017
[9] S. Elliott, A. Sinclair, H. Collins, L. Rice, and W. Jelkmann, “Progress in detecting cell-surface protein receptors: The erythropoietin receptor example,” Ann. Hematol., vol. 93, Dec. 2013
[10] F. J. Gonzalez, C. Xie, and C. Jiang, “The role of hypoxia-inducible factors in metabolic diseases,” Nat. Rev. Endocrinol., vol. 15, no. 1, pp. 21–32, 2018
[11] W. Jelkmann, “Regulation of erythropoietin production,” J. Physiol., vol. 589, no. 6, pp. 1251–1258, 2011
[12] S. Genc, T. F. Koroglu, and K. Genc, “Erythropoietin and the nervous system,” Brain Res., vol. 1000, no. 1, pp. 19–31, 2004
[13] B. B. Beleslin-Čokić et al., “Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells,” Cytokine, vol. 54, no. 2, pp. 129–135, 2011
[14] C. J. Parsa et al., “Cardioprotective Effects of Erythropoietin in the Reperfused Ischemic Heart: A Potential role for cardiac fibroblasts,” J. Biol. Chem., vol. 279, no. 20, pp. 20655–20662, May 2004
[15] S. Lamon and A. Russell, “The role and regulation of erythropoietin (EPO) and its receptor in skeletal muscle: how much do we really know?,” Frontiers in Physiology , vol. 4. 2013
[16] M. Alnaeeli and C. T. Noguchi, “Erythropoietin and obesity-induced white adipose tissue inflammation: redefining the boundaries of the immunometabolism territory,” Adipocyte, vol. 4, no. 2, pp. 153–157, Apr. 2015
[17] S. Suresh, J. Lee, and C. T. Noguchi, “Erythropoietin signaling in osteoblasts is required for normal bone formation and for bone loss during erythropoietin-stimulated erythropoiesis,” FASEB J., vol. 34, no. 9, pp. 11685–11697, Sep. 2020
[18] T. Waas et al., “Distribution of estimated glomerular filtration rate and determinants of its age dependent loss in a German population-based study,” Sci. Rep., vol. 11, no. 1, p. 10165, 2021
[19] M. C. Thomas, M. E. Cooper, C. Tsalamandris, R. MacIsaac, and G. Jerums, “Anemia With Impaired Erythropoietin Response in Diabetic Patients,” Arch. Intern. Med., vol. 165, no. 4, pp. 466–469, Feb. 2005
[20] Y. Fujita et al., “Low erythropoietin levels predict faster renal function decline in diabetic patients with anemia: a prospective cohort study,” Sci. Rep., vol. 9, no. 1, pp. 1–10, 2019
[21] W. B. Ershler et al., “Serum erythropoietin and aging: A longitudinal analysis,” J. Am. Geriatr. Soc., vol. 53, no. 8, pp. 1360–1365, 2005
[22] G. de Haan and S. S. Lazare, “Aging of hematopoietic stem cells,” Blood, vol. 131, no. 5, pp. 479–487, Feb. 2018
[23] M. J. Koury and P. Ponka, “NEW INSIGHTS INTO ERYTHROPOIESIS: The Roles of Folate, Vitamin B12, and Iron,” Annu. Rev. Nutr., vol. 24, no. 1, pp. 105–131, Jun. 2004
[24] G. E. Abraham, U. D. Schwartz, and M. M. Lubran, “Effect of vitamin B-6 on plasma and red blood cell magnesium levels in premenopausal women,” Ann. Clin. Lab. Sci., vol. 11, no. 4, pp. 333–336, 1981
[25] C. W. Wong, “Vitamin B12 deficiency in the elderly: Is it worth screening?,” Hong Kong Med. J., vol. 21, no. 2, pp. 155–164, 2015
[26] E. H. Reynolds, “Folic acid, ageing, depression, and dementia,” vol. 324, no. June, 2002
[27] L. Kautz and E. Nemeth, “Molecular liaisons between erythropoiesis and iron metabolism,” Blood, vol. 124, no. 4, pp. 479–482, Jul. 2014
[28] M. C. Dao and S. N. Meydani, “Iron Biology, Immunology, Aging, and Obesity: Four Fields Connected by the Small Peptide Hormone Hepcidin,” Adv. Nutr., vol. 4, no. 6, pp. 602–617, Nov. 2013
[29] D. Mangan, “Iron: an underrated factor in aging,” Aging (Albany. NY)., vol. 13, no. 19, pp. 23407–23415, Oct. 2021
[30] S. J. Fairweather-Tait, A. A. Wawer, R. Gillings, A. Jennings, and P. K. Myint, “Iron status in the elderly,” Mech. Ageing Dev., vol. 136–137, pp. 22–28, 2014
[31] B. J. Cherayil, “Iron and immunity: immunological consequences of iron deficiency and overload,” Arch. Immunol. Ther. Exp. (Warsz)., vol. 58, no. 6, pp. 407–415, Dec. 2010