In a recent Perspective published in Nature Aging, researchers noted a lack of studies on female-specific physiological factors on aging trajectories. They proposed solutions, including model systems, to address the issue [1].
Most model organisms don’t match human sex specificity
The use of model organisms, such as worms, fruit flies, and mice, has helped researchers to understand many aging processes. However, it is also partly to blame for the lack of data on how pregnancy, breastfeeding, and menopause impact aging-related diseases.
Animal research is based on the premise that those organisms and humans share essential physiological characteristics. While true in many cases, there are tremendous differences between the physiology of model organisms and female reproductive biology.
One example relates to bone mass. The bone mass of female rodents does not decline more than that of male rodents [2], while human females suffer from osteoporosis four times more frequently than males [3]. Similarly, sarcopenia is observed in human females earlier than in males [4], while in rodents, sex differences are almost non-existent [5].
The authors also bring up Alzheimer’s disease and non-Alzheimer’s dementia, which in human females is higher compared to age-matched males [6]. This is not the case for rodents, as studies have observed better retained memory-related functions over time in female rodents when compared to age-matched male rodents [7].
While those are just some well-documented examples, the authors emphasize that it’s likely that many more exist. However, they are unnoticed due to the prevalence of using male models to study aging [8]. This has led to a gap in knowledge regarding female-specific aging processes and disease onset and progression. The authors link this gap to worse health outcomes in the female population, such as females living with higher morbidity when compared to age-matched males despite living longer on average [9, 10].
Females are also more frequently misdiagnosed, with heart attacks being 50% more likely to be misdiagnosed in females [11]. Currently available drugs for age-related diseases are also more likely to cause side effects in females [12].
Understanding the gap
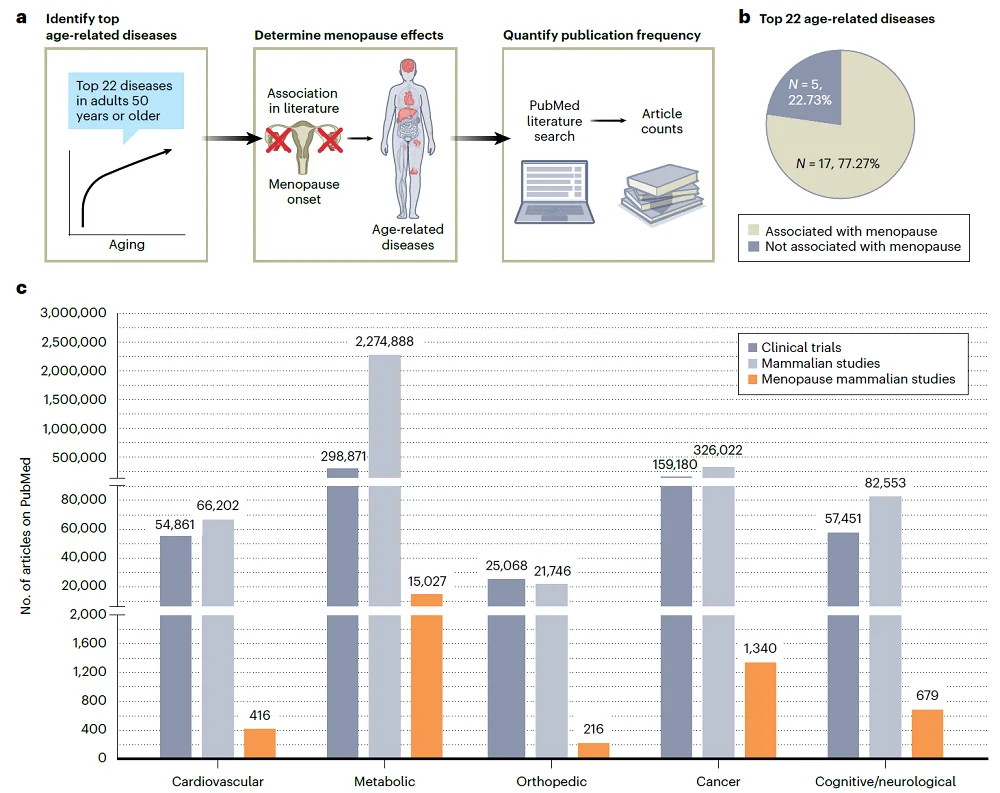
To understand the extent to which female reproductive biology is considered in aging research using model organisms, the authors performed a literature search to identify the number of “clinical trials, mammalian studies and mammalian studies that considered menopause in aging research” focusing on the 22 most prevalent diseases among older adults.
Analyzing the literature, the authors made several interesting observations. “Over 70% of the most prevalent age-related diseases are associated with and/or potentially impacted by menopause.“ Even though this number is so high, authors noted that only 1% of preclinical studies they identified in their search considered menopause.
Menopause is not the only overlooked component of female biology in aging studies. Pregnancy, birthing, and breastfeeding are also commonly not factored in typical research, even though these life events are widely experienced and are known to impact longevity and healthspan.
Research has found that childbirth and breastfeeding can affect the health of an individual in both positive and negative ways. For example, pregnancy complications increase cardiovascular and metabolic risk [13]. On the other hand, breastfeeding can positively impact women’s health, as it is associated with lowering the likelihood of breast and ovarian cancer risk [14, 15], type 2 diabetes [16], and high blood pressure later in life [17].
New models for female aging research
In their effort to provide solutuons, the authors listed models that, while having their shortcomings, can be used to reflect the impact of menopause or pregnancy, such as by performing surgical removal of ovaries (ovariectomy) on mice, or the use of VCD, a toxin that causes ovarian follicle apoptosis [18].
The authors also mention a newly described model, Acomys cahirinus, the African spiny mouse. These mice are the first ones to be reported to menstruate and to undergo similar menopause to that of humans [19]. They also suggest using mice that were used for breeding in the experiments and not, as is currently done, those that never produced a litter. Additionally, they suggest that the sex of a tissue donor should be mentioned in cell culture experiments.
Room for improvement
In their paper, the authors offer recommendations for researchers, scientific journals, providers of animals for aging research, and funding agencies on how they can address this issue.
The most obvious recommendation is simply for researchers to embark more on research that addresses the impact of menopause, pregnancy, and breastfeeding on aging and disease progression and use proper animal models and experimental setups, thus improving how their studies handle the particular ways in which females age.
Similarly, the authors recommend that funders ameliorate this problem by providing financial support for research that specifically monitors sex-dependent differences in experimental outcomes. Journals can ensure that the reporting of sex-specific data is done properly, and providers of animals can make necessary animal models readily available for researchers.
Literature
[1] Gilmer, G., Hettinger, Z. R., Tuakli-Wosornu, Y., Skidmore, E., Silver, J. K., Thurston, R. C., Lowe, D. A., & Ambrosio, F. (2023). Female aging: when translational models don’t translate. Nature aging, 3(12), 1500–1508.
[2] Sophocleous, A., & Idris, A. I. (2014). Rodent models of osteoporosis. BoneKEy reports, 3, 614.
[3] Alpantaki, K., Papadaki, C., Raptis, K., Dretakis, K., Samonis, G., & Koutserimpas, C. (2020). Gender and Age Differences in Hip Fracture Types among Elderly: a Retrospective Cohort Study. Maedica, 15(2), 185–190.
[4] Kurose, S., Nishikawa, S., Nagaoka, T., Kusaka, M., Kawamura, J., Nishioka, Y., Sato, S., Tsutsumi, H., & Kimura, Y. (2020). Prevalence and risk factors of sarcopenia in community-dwelling older adults visiting regional medical institutions from the Kadoma Sarcopenia Study. Scientific reports, 10(1), 19129.
[5] Clemens, Z., Sivakumar, S., Pius, A., Sahu, A., Shinde, S., Mamiya, H., Luketich, N., Cui, J., Dixit, P., Hoeck, J. D., Kreuz, S., Franti, M., Barchowsky, A., & Ambrosio, F. (2021). The biphasic and age-dependent impact of klotho on hallmarks of aging and skeletal muscle function. eLife, 10, e61138.
[6] Beam, C. R., Kaneshiro, C., Jang, J. Y., Reynolds, C. A., Pedersen, N. L., & Gatz, M. (2018). Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD, 64(4), 1077–1083.
[7] Mifflin, M. A., Winslow, W., Surendra, L., Tallino, S., Vural, A., & Velazquez, R. (2021). Sex differences in the IntelliCage and the Morris water maze in the APP/PS1 mouse model of amyloidosis. Neurobiology of aging, 101, 130–140.
[8] Carmody, C., Duesing, C. G., Kane, A. E., & Mitchell, S. J. (2022). Is Sex as a Biological Variable Still Being Ignored in Preclinical Aging Research?. The journals of gerontology. Series A, Biological sciences and medical sciences, 77(11), 2177–2180.
[9] Boerma, T., Hosseinpoor, A. R., Verdes, E., & Chatterji, S. (2016). A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC public health, 16, 675.
[10] Singh-Manoux, A., Guéguen, A., Ferrie, J., Shipley, M., Martikainen, P., Bonenfant, S., Goldberg, M., & Marmot, M. (2008). Gender differences in the association between morbidity and mortality among middle-aged men and women. American journal of public health, 98(12), 2251–2257.
[11] Wong, C. W., Tafuro, J., Azam, Z., Satchithananda, D., Duckett, S., Barker, D., Patwala, A., Ahmed, F. Z., Mallen, C., & Kwok, C. S. (2021). Misdiagnosis of Heart Failure: A Systematic Review of the Literature. Journal of cardiac failure, 27(9), 925–933.
[12] Franconi, F., Brunelleschi, S., Steardo, L., & Cuomo, V. (2007). Gender differences in drug responses. Pharmacological research, 55(2), 81–95.
[13] Neiger R. (2017). Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. Journal of clinical medicine, 6(8), 76.
[14] Collaborative Group on Hormonal Factors in Breast Cancer (2002). Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet (London, England), 360(9328), 187–195.
[15] Babic, A., Sasamoto, N., Rosner, B. A., Tworoger, S. S., Jordan, S. J., Risch, H. A., Harris, H. R., Rossing, M. A., Doherty, J. A., Fortner, R. T., Chang-Claude, J., Goodman, M. T., Thompson, P. J., Moysich, K. B., Ness, R. B., Kjaer, S. K., Jensen, A., Schildkraut, J. M., Titus, L. J., Cramer, D. W., … Terry, K. L. (2020). Association Between Breastfeeding and Ovarian Cancer Risk. JAMA oncology, 6(6), e200421.
[16] Morris A. (2018). Risk factors: Breastfeeding reduces risk of type 2 diabetes mellitus. Nature reviews. Endocrinology, 14(3), 128.
[17] Park, S., & Choi, N. K. (2018). Breastfeeding and Maternal Hypertension. American journal of hypertension, 31(5), 615–621.
[18] Kappeler, C. J., & Hoyer, P. B. (2012). 4-vinylcyclohexene diepoxide: a model chemical for ovotoxicity. Systems biology in reproductive medicine, 58(1), 57–62.
[19] Bellofiore, N., Ellery, S. J., Mamrot, J., Walker, D. W., Temple-Smith, P., & Dickinson, H. (2017). First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). American journal of obstetrics and gynecology, 216(1), 40.e1–40.e11.



