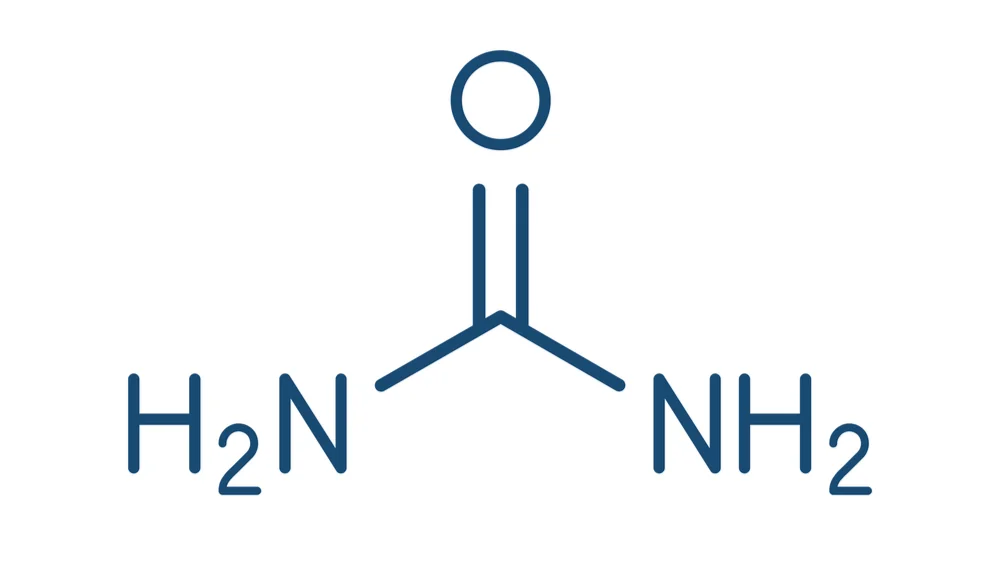
Urea Is a Blood Biomarker of Aging
In 2013, Steve Horvath developed a highly accurate artificial intelligence-driven method of determining biological age [1]. This long-awaited development ushered in a new era of aging research. For the first time, it enabled researchers in academia and industry to directly measure some of the results of their work without waiting for lifespans to end.
For example, in 2019, Dr. Greg Fahy conducted an experiment aimed at thymus gland regeneration. There were nine participants in the year-long study. In addition to partial regeneration of the participants’ thymus glands, their average biological age based on DNA methylation patterns decreased by 2.5 years [2].
Since then, researchers have developed many AI-based methods for assessing biological age and mortality. Mortality is defined as the rate of death in a given population during a specified interval [3]. A brief glance at aging-related studies at clinicaltrials.gov reveals the wide variety of interventions currently under study.
Public access and self-study
Researchers have made these biological age assessment tools accessible to the public, giving individuals the opportunity to self-experiment with diet, lifestyle, exercise, supplementation, and other measures.
One such avenue of biological age testing that is low cost, accessible, and accurate is provided by Levine’s Phenotypic Age Calculator and Aging.ai’s 1.0, 2.0, and 3.0 assays. These estimators simply require the user to enter standardized blood test values into an online platform and hit the send button. Some insurance companies cover this blood testing.
The need for biomarkers
Biomarker values provides a starting point that yields insights into individual physiology and how lifestyle changes, tracked over time, impact it. Urea ranks fifth in relative importance among Aging.ai’s big five blood biomarkers. The other four are albumin, alkaline phosphatase, glucose, and red blood cells.
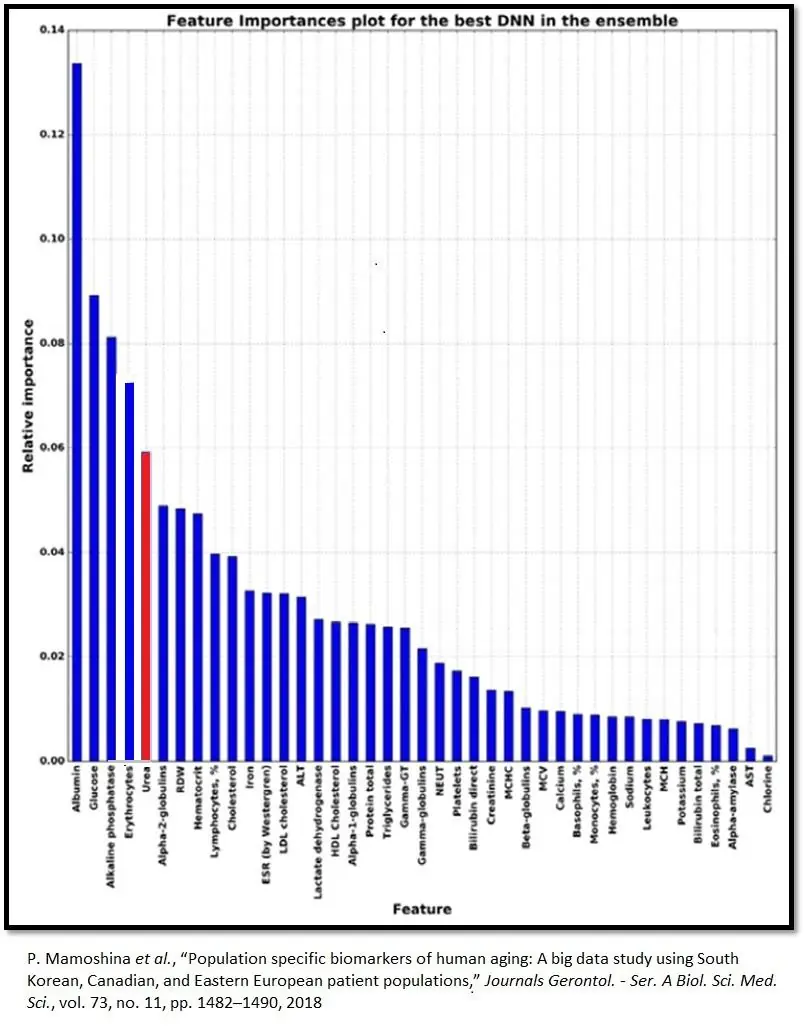
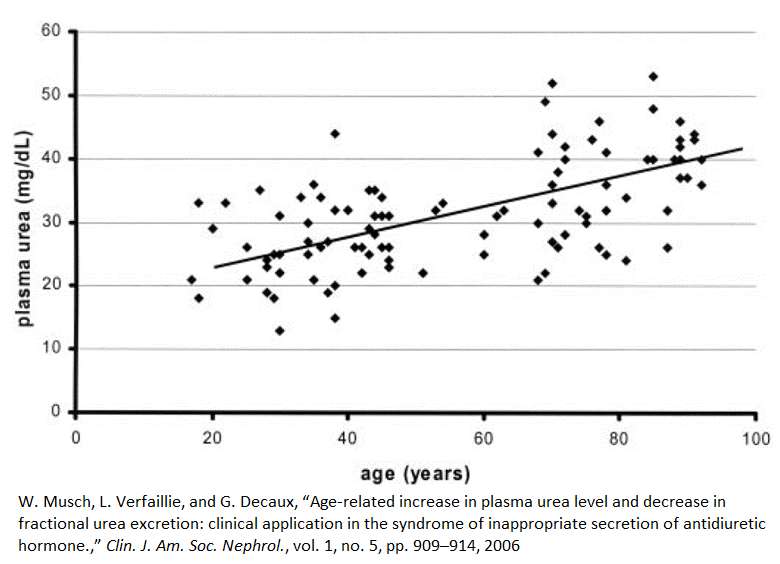
Plasma urea concentrations rise with age
Between the ages of 20 and 80, plasma urea concentrations, on average, increase by 60%, or 1% per year [4]. This graph shows that some 80-year-olds have urea plasma concentrations that nearly match those of the average 20-year-old. On the other hand, some people under the age of 40 have the plasma urea concentrations of an average 90-year-old.
There is some evidence that our genes play a large role in our longevity. However, a pivotal 1996 study of Danish twins has called that assumption into question [5]. This study concluded that more than 90 percent of longevity is determined by choices instead of genetics.
People can have a genetic predisposition for certain diseases, but their bodies may not express the gene or set of genes associated with the more rapid decline of a tissue, organ, or system if they are not triggered by specific epigenetic factors. Epigenetic factors, like methylation, are a major determinant of which specific genes the body expresses in each cell. It turns out epigenetic factors a quite responsive to environmental conditions that, most often, we can control [5].
Urea and its toxicity
In chronic kidney disease, damaged kidneys cause the accumulation of urea by slowing down its elimination [4]. Doctors routinely measure plasma urea to assess kidney function. Recent evidence suggests that urea is a potent toxin with multiple systemic effects [6].
The body makes proteins by bonding together long chains of amino acids. When the body has an excess of protein, is in the process of turning over cellular proteins, or has a deficit of available carbohydrate or fat to meet its energy needs, it activates the urea cycle to deconstruct proteins and their amino acid constituents [7].
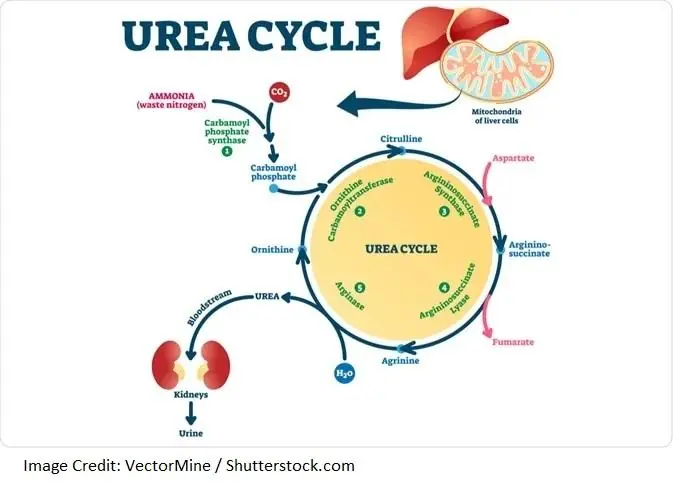
Part of this process, called deamination, is the removal of amine group(s) (NH2) from amino acids. The deamination process produces highly toxic ammonia (NH3), which the body deals with by converting it to urea, a less toxic byproduct [7].
Even though urea is protective against ammonia toxicity, evidence suggests that rising urea levels damage the body across multiple systems. Urea-related damage can occur as a direct result of urea or indirectly through the breakdown of urea to isocyanic acid (carbamylation) [8].
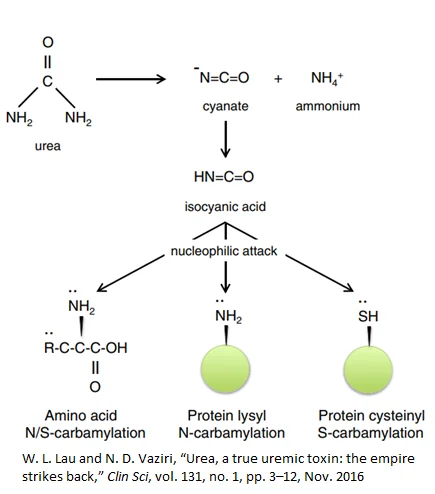
Isocyanic acid interacts with, and irreversibly damages, cysteine and lysine side-chains on proteins, potentially rendering them useless and producing potent downstream effects such as anemia [9], renal fibrosis [10], and atherosclerosis. In fact, elevated levels carbamylated proteins in the blood of dialysis patients predicts higher death risk [11].
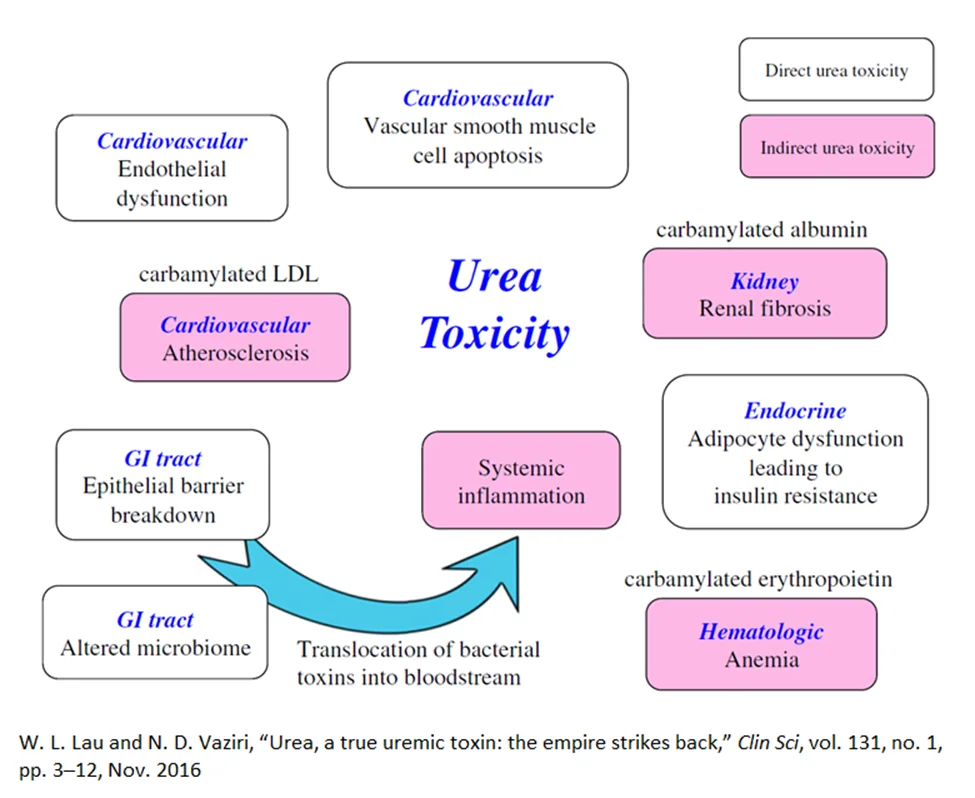
Urea, directly or indirectly, can affect the intestinal tract, kidneys, and cardiovascular system, and it can disrupt glucose metabolism [10].
In the intestinal tract, elevated levels of urea alter the normal distribution of gut microbes and disrupt normal barrier function [12, 13]. This results in the inability of the gut wall to screen out bacterial toxins, such as indoxyl sulfate and p-cresyl sulfate [14, 15].
The influx of bacterial toxin into the bloodstream results in systemic toxicity which is linked with the progression of kidney failure and cardiovascular death. In the kidney, studies have shown that elevated urea levels drive increased abnormal collagen deposition (fibrosis) [6, 14].
Evidence indicates that elevated urea directly drives atherosclerosis by causing arterial wall dysfunction and vascular smooth muscle apoptosis. Indirectly, the carbamylation process alters LDL, which increases its propensity to damage the arterial wall and cause vascular calcification [16, 17].
Urea has also been shown to impair the response of fat cells to insulin, leading to impaired glucose metabolism. Lastly, carbamylation damages the hormone erythropoietin, which can lead to anemia [6, 10].
There is limited evidence that the rate of urea clearance may not be of any great consequence, but the studies used to support this assertion are arguably outdated and flawed in a number of other ways. Most of these studies were conducted on dialysis patients with prolonged urea elevations and advanced kidney disease [18, 19]. Further, dialysis itself promotes inflammation, which is likely to obscure the benefits of urea clearance [20, 21].
Additionally, conventional methods for measuring urea clearance are oversimplified and do not consider that many other toxins as well as water/salt balance affect clinical outcomes. Lastly, blood urea levels are affected by kidney function, protein intake, hydration state, and the presence of internal bleeding [21].
The most persuasive evidence that lowering urea levels is beneficial comes from studies examining the effects of low-protein diets, which lower the production of urea and slow the progressive loss of kidney function. Several studies have shown that a carefully monitored low-protein diet that avoids malnutrition can be protective for kidney function and delay progression to end-stage kidney failure [22].
Combating rising urea levels
Plasma urea levels primarily rise due to a decline in excretion rather than an increase in production [4]. Subclinical elevations in urea with increasing chronological age often reflect modifiable variables, including protein consumption [22], hydration [23], blood pressure [24], glucose levels [25], and fiber intake [26-28].
Increasing hydration to optimal levels can significantly reduce plasma urea [29, 30]. People who work in hot environments or lose a significant amount of body water through exercise should adhere to the following guidelines [31]:
- Drink 17-20 ounces of water two to three hours before the start of exercise
- Drink 8 ounces of fluid 20 to 30 minutes prior to exercise or during warm-up
- Drink 7-10 ounces of fluid every 10 to 20 minutes during exercise
- Drink an additional 8 ounces of fluid within 30 minutes after exercising
- Drink 16-24 ounces of fluid for every pound of body weight lost after exercise
Individual protein needs can vary considerably depending on genetics, lifestyle factors, and personal goals. A 2018 study examining evidence from 17 studies with 2,996 people with reduced kidney function concluded that very low-protein diets, compared to low or normal protein intake, reduce urea production, which reduces plasma urea and so reduces the risk of advanced kidney failure that progress to dialysis [32]. The key is to meet protein requirements without consuming excessive protein.
Common recommendations for maintaining healthy blood pressure include regular exercise, avoidance of high-sodium foods, excessive alcohol consumption, excessive stress, poor sleeping habits, and smoking [33]. Basic methods of controlling blood glucose include monitoring glucose levels, getting regular exercise, and eating healthy fats and high-fiber foods [34].
Traditionally, doctors have often limited fiber in patients with advanced kidney disease because high-fiber foods tend to also be high in potassium, and damaged kidneys do not eliminate potassium particularly well; this can be lethal. However, most peopel do not consume adequate fiber or potassium [35]. A recent meta-analysis of 14 clinical trials found that dietary fiber supplementation significantly reduced serum urea and creatinine levels [36]. Therefore, people without chronic kidney disease can lower urea through fiber consumption, but people who have the disease should monitor their potassium levels and consult their physicians about optimal fiber intake.
Literature
[1] S. Horvath, “DNA methylation age of human tissues and cell types,” Genome Biol, vol. 14, no. 10, p. 3156, 2013
[2] G. M. Fahy et al., “Reversal of epigenetic aging and immunosenescent trends in humans,” Aging Cell, vol. 18, no. 6, p. e13028, Dec. 2019
[3] J. Hernandez and P. Kim, Epidemiology Morbidity And Mortality. Treasure Island (Fl): StatPearls Publishing, 2022.
[4] W. Musch, L. Verfaillie, and G. Decaux, “Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone.,” Clin J Am Soc Nephrol, vol. 1, no. 5, pp. 909–914, 2006
[5] A. M. Herskind, M. McGue, N. v Holm, T. I. A. Sørensen, B. Harvald, and J. W. Vaupel, “The heritability of human longevity: A population-based study of 2872 Danish twin pairs born 1870–1900,” Hum Genet, vol. 97, no. 3, pp. 319–323, 1996
[6] W. L. Lau and N. D. Vaziri, “Urea, a true uremic toxin: the empire strikes back,” Clin Sci, vol. 131, no. 1, pp. 3–12, Nov. 2016
[7] W. Barmor, F. Azad, and W. Stone, “Physiology, Urea Cycle – StatPearls – NCBI Bookshelf.”
[8] G. R. Stark, “Reactions of cyanate with functional groups of proteins. II. Formation, decomposition, and properties of N-carbamylimidazole,” Biochemistry, vol. 4, no. 3, pp. 588–595, Mar. 1965
[9] R. Flückiger, W. Harmon, W. Meier, S. Loo, and K. H. Gabbay, “Hemoglobin carbamylation in uremia,” N. Engl. J. Med., vol. 304, no. 14, pp. 823–827, Apr. 1981
[10] M. D’Apolito et al., “Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure,” Journal of Clinical Investigation, vol. 120, no. 1, pp. 203–213, Jan. 2010
[11] E. Ok, A. G. Basnakian, E. O. Apostolov, Y. M. Barri, and S. v. Shah, “Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease,” Kidney Int., vol. 68, no. 1, pp. 173–178, Jul. 2005
[12] D. F. Birt et al., “Resistant Starch: Promise for Improving Human Health,” Advances in Nutrition, vol. 4, no. 6, pp. 587–601, Nov. 2013
[13] D. A. Kieffer et al., “Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats,” Am J Physiol Renal Physiol, vol. 310, no. 9, pp. F857–F871, May 2016
[14] M. Bossola et al., “Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients,” Clin J Am Soc Nephrol, vol. 4, no. 2, pp. 379–385, Feb. 2009
[15] U. Feroze et al., “Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients,” J Ren Nutr, vol. 22, no. 3, pp. 317–326, May 2012, doi: 10.1053/J.JRN.2011.05.004.
[16] A. Covic et al., “Vascular calcification in chronic kidney disease,” Clin Sci (Lond), vol. 119, no. 3, pp. 111–121, Aug. 2010
[17] K. Nitta, “Vascular calcification in patients with chronic kidney disease,” Therapeutic Apheresis and Dialysis, vol. 15, no. 6, pp. 513–521, Dec. 2011
[18] R. A. A. Paniagua et al., “Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial,” J Am Soc Nephrol, vol. 13, no. 5, pp. 1307–1320, May 2002
[19] J. W. Yoon, M. v. Pahl, and N. D. Vaziri, “Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease,” Kidney Int, vol. 71, no. 2, pp. 167–172, Jan. 2007
[20] J. J. Carrero et al., “Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM),” J Ren Nutr, vol. 23, no. 2, pp. 77–90, Mar. 2013
[21] Wei Ling Lau, “Urea is truly toxic: the empire strikes back – Atlas of Science.”
[22] D. Hahn, E. M. Hodson, and D. Fouque, “Low protein diets for non-diabetic adults with chronic kidney disease,” Cochrane Database of Systematic Reviews, vol. 2020, no. 11, Oct. 2020
[23] “Can water intake prevent CKD? A brief review of the evidence.”
[24] W. R. Zhang et al., “Kidney damage biomarkers and incident chronic kidney disease during blood pressure reduction: A case-control study,” Ann Intern Med, vol. 169, no. 9, pp. 610–618, Nov. 2018
[25] M. Ruospo et al., “Glucose targets for preventing diabetic kidney disease and its progression,” Cochrane Database of Systematic Reviews, vol. 2017, no. 6, Jun. 2017.
[26] N. D. Vaziri, “CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity,” Curr Opin Nephrol Hypertens, vol. 21, no. 6, p. 587, Nov. 2012
[27] Y. Guo et al., “Impaired intestinal barrier function in a mouse model of hyperuricemia,” Mol Med Rep, vol. 20, no. 4, pp. 3292–3300, Oct. 2019
[28] T. van Hung and T. Suzuki, “Dietary Fermentable Fibers Attenuate Chronic Kidney Disease in Mice by Protecting the Intestinal Barrier,” J Nutr, vol. 148, no. 4, pp. 552–561, Apr. 2018
[29] R. Rylander and M. J. Arnaud, “Mineral water intake reduces blood pressure among subjects with low urinary magnesium and calcium levels,” BMC Public Health, vol. 4, Nov. 2004
[30] N. di Paolo, G. A. Nicolai, M. Lombardi, F. Maccari, and G. Garosi, “High doses of water increase the purifying capacity of the kidneys,” International Journal of Artificial Organs, vol. 30, no. 12, pp. 1109–1115, 2007
[31] “Report Sets Dietary Intake Levels for Water, Salt, and Potassium To Maintain Health and Reduce Chronic Disease Risk | National Academies.” February 2004
[32] D. Hahn, E. M. Hodson, and D. Fouque, “Low protein diets for non-diabetic adults with chronic kidney disease,” Cochrane Database of Systematic Reviews, vol. 2020, no. 11, Oct. 2020
[33] “10 ways to control high blood pressure without medication – Mayo Clinic.”
[34] “Diabetes prevention: 5 tips for taking control – Mayo Clinic.”
[35] A. Ascherio et al., “Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men,” Circulation, vol. 98, no. 12, 1998
[36] L. Chiavaroli, A. Mirrahimi, J. L. Sievenpiper, D. J. A. Jenkins, and P. B. Darling, “Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials,” European Journal of Clinical Nutrition 2015 69:7, vol. 69, no. 7, pp. 761–768, Nov. 2014