The Relationship Between ApoE and Alzheimer’s
Alzheimer’s disease is the most typical form of dementia. It is a progressive disease that begins with mild memory loss, leads to profound cognitive deficits, and ends with death. 10% to 14% of adults over 65 have some form of Alzheimer’s. There is no known cure. Carriers of the APOE4 gene have a significantly greater likelihood of developing Alzheimer’s disease [1].
A brief history of APOE
APOE4 was the first of three genetic isoforms of the APOE gene to emerge on the evolutionary landscape. Theorists have proposed that APOE4 is very efficient at promoting acute localized inflammatory responses [2]. This conferred a significant survival advantage in an age when sepsis kept life expectancy at 30 years and infant mortality near 30% [3].
As the human species migrated from Africa to Europe and Asia, significant changes in diet and lifestyle led to a fall in the incidence of sepsis. Infections took a back seat to heart disease, cancer, and, eventually, neurodegenerative conditions such as Alzheimer’s disease [2].
Changes in the leading causes of death reduced evolutionary pressure to conserve the APOE4 gene and thus enabled the emergence of the APOE2 and APOE3 variants [4-6]. In the early 1990s, Allen Roses and his collaborators at Duke University challenged the prevailing theories on the development of Alzheimer’s. Roses presented evidence from large data sets that pointed to APOE4 as a primary driver of Alzheimer’s pathology [7].
In the years since, researchers have also uncovered evidence that APOE4 carriers may also have an increased risk of cardiovascular disease and reduced longevity [8]. In part, the reduction in longevity appears to be independent of both Alzheimer’s and cardiovascular disease [9].
APOE and its associated proteins
Research is gradually uncovering the mechanisms by which APOE4 drives the development of Alzheimer’s disease, cardiovascular pathology, and reduced longevity. The APOE4 gene and its isoforms APOE3 and APOE2 provide instructions for making the corresponding sets of ApoE apolipoproteins.
Like the genes that express them, the ApoE protein isoforms have slight differences. All ApoE variants consist of a sequence of 299 amino acids. These proteins are glycosylated, which means that specifically placed molecules of sugar add functionality to the protein. The distinguishing differences between ApoE2, ApoE3, and ApoE4 are the amino acids located at positions 112 and 158. Although the variants are 99.3% identical, these amino acid substitutions have immense functional consequences [10].
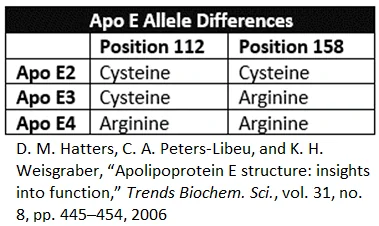
How ApoE functions systemically
ApoE is a component of lipoproteins, which are vesicles that carry cholesterol and triglycerides through the bloodstream. LDL, HDL, VLDL, and chylomicrons are all lipoproteins.
Lipids are chemicals that don’t readily mix with water. However, the body makes lipids and absorbs them from food. The body uses these lipids for structural components, energy, and chemical signaling. Since fluid in the circulatory systems is water-based, it is necessary to organize lipids into water-soluble structures in order to transport them. One way is by making lipoproteins [11,12].
Lipoproteins are largely self-assembled structures that form inside certain cells. The surface of a lipoprotein consists of apolipoproteins (such as ApoE), phospholipids, and cholesterol. These three elements form an outer water-friendly shell that enables the transport of lipid-filled vesicles through the watery environment of the body. The interior of the lipoprotein is filled with triglycerides and cholesterol [11,12].
ApoE is not the only apolipoprotein, but it is distinct from its cousins because it can assume multiple forms and functions. ApoE has two different domains, which are areas of the protein that interact with other molecules. ApoE has a receptor-binding domain and a lipid-binding domain. These domains interact with cells, other lipids, and amyloid beta in many ways [13].
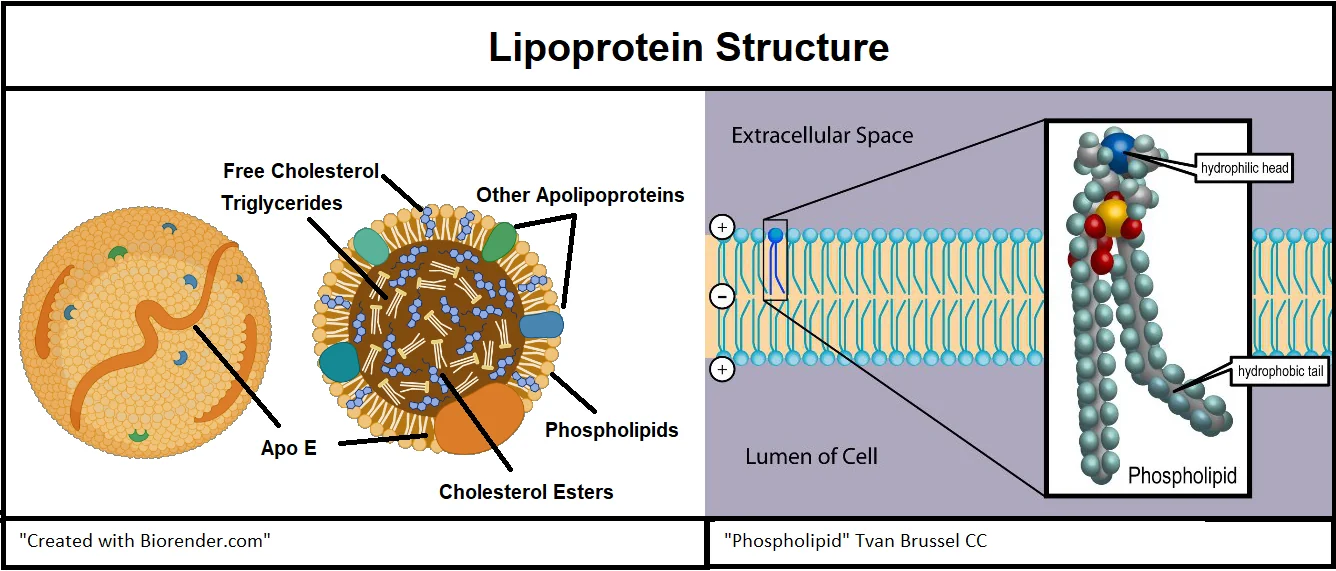
Each of the ApoE variants performs these functional interactions differently, however. In many instances, ApoE4 suboptimally performs these functions compared to ApoE3 and ApoE2 variants, as there are many unfavorable outcomes associated with ApoE4 [10].
Phospholipids have a lipid-soluble end and a water-soluble end. When ApoE is functioning as a lipoprotein constituent, the lipid-binding portion faces inward to bind to the lipid portion of the phospholipids, and the water-soluble receptor portion faces out of the lipoprotein, where it can interact with receptors on the surface of cells that the lipoprotein may encounter [14].
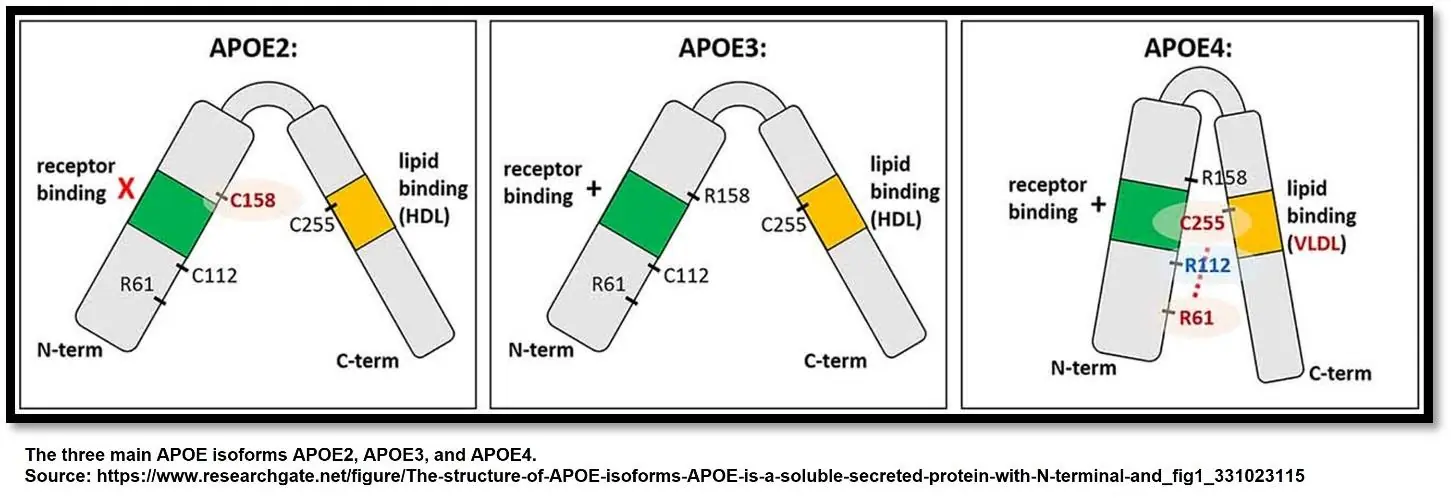
ApoE in Alzheimer’s disease and amyloid beta clearance
Brain tissue affected by Alzheimer’s disease is laden with amyloid beta aggregates outside of neurons and neurofibrillary tangles inside them. This interferes with normal nerve conduction and kills neurons, leading to the progressive loss of cognitive function and, ultimately, death [15]. The extent to which amyloid beta aggregates and neurofibrillary tangles form is strongly related to APOE genetic variants. APOE4 carriers are much more likely to develop Alzheimer’s [16].
APOE4 is also associated with cardiovascular disease and reduced longevity. Conversely, APOE2 carriers have a greatly reduced likelihood of developing Alzheimer’s and a lower risk of developing cardiovascular disease, and they tend to have longer lifespans [8,9].
Image source: Alzheimers Disease by Bruce Blaus License: Creative Commons and Attribution-Share Alike 4.0 International licenses. Declaration: This image has not been modified
ApoE in the brain
ApoE interacts with amyloid beta in both competitive and cooperative ways at cell surface receptors in the brain [17]. The main cell surface receptors LDL, LRP1, and HSPG are present on neurons, astrocytes, microglia, and oligodendrocytes, and they mediate the uptake of ApoE and amyloid beta. However, ApoE2, ApoE3, and ApoE4 all behave differently from one another during this mediation process. Further, the binding site occupation of each ApoE isoform alters its behavior significantly [16].
Brain receptor activity in Alzheimer’s disease
Cell surface receptors, such as the LDL receptor, do different things in different contexts. In one context, LDL receptors act as relays in cellular signaling cascades. In another context, these receptors function as a docking port that enables the transfer of lipids and cholesterol between lipoproteins and cells [18].
Part of the diverse array of ApoE’s receptor interactions are due to changes in the shape of different ApoE isoforms that occur when the lipid-binding domain becomes occupied. Occupation can occur because of lipid binding or the binding of other molecules, such as HSPG, which has the unique ability to bind both of ApoE’s domains [19].
LDL receptors
High concentrations of LDL receptors reduce ApoE levels by 50 to 90%. Unlike ApoE4, ApoE2 has a very low affinity for LDL receptors [20].
If lipids occupy the lipid-binding domain of ApoE3 and ApoE4, they readily bind to LDL receptors because these receptors are driven to bind to the lipid cargo that ApoE has sequestered [21]. If the lipid-binding domain is unoccupied, binding affinity decreases due to a change in the shape of the ApoE protein [16].
Because ApoE4 has a very high affinity for the LDL receptor [20], the cell rapidly removes ApoE4 from the intercellular space via endocytosis. The absence of ApoE4 from the intercellular space promotes the accumulation of amyloid beta. This is because ApoE present in the intercellular space can complex with amyloid beta peptides and shuttle them across the blood-brain barrier. Enzymes outside of the brain further degrade amyloid beta [22]. To the extent that ApoE4 remains in the intercellular space, it does not bind to amyloid beta with the same affinity that ApoE2 does [16].
ApoE4 also competes with amyloid beta for uptake by astrocytes. As astrocytes take up ApoE4, amyloid beta accumulates in the intercellular space, setting the stage for the formation of amyloid plaques [23]. Therefore, ApoE4’s affinity for the LDL receptor, its reduced ability to bind to amyloid beta, and its competition with amyloid beta for uptake by astrocytes account for ApoE4’s suboptimal ability to facilitate amyloid beta clearance in significant measure.
This is consistent with research demonstrating that ApoE2 carriers have higher concentrations of ApoE2 in cerebrospinal fluid (CSF) and plasma than ApoE3 or ApoE4 carriers. ApoE4 carriers have the lowest concentrations of ApoE in CSF and plasma [24].
LRP1 and sLRP1
LRP1 is related to the LDL receptor. In addition to its presence on cells, researchers have found a soluble form of LRP1 referred to as sLRP1. Both forms of LRP1 can interact with ApoE and amyloid beta. LRP1 recognizes more than 30 structurally and functionally distinct ligands, including apoE, α2-macroglobulin, tissue-type plasminogen activator, APP and amyloid beta [16,25].
sLRP1 floats between cells, where it normally sequesters 70-90% of plasma amyloid beta peptides. Following sequestration, sLRP1 transports amyloid beta across the blood-brain barrier removing it from the brain [26].
It is known, however, that aging and Alzheimer’s are associated with decreased LRP1 and sLRP1 expression [27]. This is known to result in reduced clearance of amyloid beta through the blood-brain barrier and reduced uptake of cholesterol by neurons.
Decreased LRP1 receptors on the surface of neurons results in an inadequate supply of cholesterol to meet the neurons’ needs. This happens because ApoE binds to the LRP1 receptor to deliver cholesterol to neurons [27].
Heparan sulfate proteoglycan (HSPG) receptor
HSPG is unlike other receptors. It binds to both receptors on ApoE: first the lipid-binding domain, then the receptor domain [28].
HSPG also has an uptake pathway that is coordinated with LRP1. ApoE first binds to HSPG and is then transferred to LRP1 for subsequent uptake, or it can bind directly to the HSPG/LRP1 complex. All three isoforms of ApoE appear to bind with HSPG with roughly the same affinity [29].
However, ApoE3 and ApoE4 bind with greater affinity to the LRP1 than ApoE2 binds to LRP1 [20]. This implies that the HSPG-LRP1 uptake pathway is not utilized by ApoE2 to the same extent as ApoE3 and ApoE4. ApoE4 would be expected to remove more amyloid beta through this mechanism than ApoE2; however, this is just one of several mechanisms by which ApoE isoforms remove amyloid beta from the brain. ApoE2 performs much better in carrying out the other mechanisms of amyloid beta removal, which offers an explanation as to why ApoE2 carriers have a much lower incidence of Alzheimer’s than ApoE3 or ApoE4 carriers [16].
There is one known risk to having two copies of the APOE2 gene: a 1 in 5000 to 1 in 10000 chance of developing a dangerous condition known as Type III Hyperlipoproteinemia [30,31]. Most often, individuals who carry two copies of the APOE2 gene have low LDL, high HDL, and triglycerides on the high side of normal, which puts them at significantly lower risk of developing heart disease and Alzheimer’s disease [8,21].
Low-molecular-weight heparin treatment inhibits binding of amyloid beta to HSPG and decreases amyloid beta concentration and deposition in the brains of mice. If LDLR or LRP1 is deleted, amyloid beta deposition is exacerbated [32]. This suggests that low-molecular-weight heparin depends on LDLR and LRP1 to remove amyloid beta from the brain and that this treatment would work better on carriers of APOE3 and APOE4 than carriers of APOE2, who occasionally do get Alzheimer’s disease.
APOE and the risk of cardiovascular disease
A 2016 meta-analysis of 30 published studies including 11,804 CHD patients and 17,713 controls examined incidence of coronary heart disease among different APOE genotypes within the general population and within Caucasian and Asian populations [33].
Study results indicated that E3/E4 and E4/E4 genotypes across an ethnically diverse sample were 22% and 45% more likely to develop CHD than E3/E3 genotypes. When the researchers controlled for ethnicity, Caucasians and Asians who carried the E4 gene were 1.25 and 2.29 times respectively more likely to develop CHD than those who carried only the E3 gene [33].
Caucasians, but not Asians, who carried the APOE2 gene were 16% less likely to develop CHD than their respective cohorts who carried the E3/E3 genotype [33].
How ApoE drives the development of cardiovascular disease
Different ApoE isoforms handle lipid transport in diverse ways. ApoE2 carriers have low LDL, high HDL, and mildly elevated triglycerides [21]. LDL transports cholesterol to the arteries, and when the LDL level is elevated, this cholesterol can accumulate in the blood vessel walls and contribute to the formation of plaques [34]. HDLs oppose atherosclerosis by removing cholesterol from foam cells present in the arterial wall and by inhibiting the oxidation of LDLs. This limits the inflammatory processes that drive atherosclerosis.
Additionally, HDL also has anti-clotting properties, which protect against strokes, heart attacks, and pulmonary embolisms [35]. The research community does not yet clearly understand the mechanistic role of triglycerides in the development of atherosclerosis. This is largely irrelevant to the ApoE isoforms because there is not a strong correlation between any of the APOE isoforms and significantly elevated triglycerides (>200mg/dl) [36].
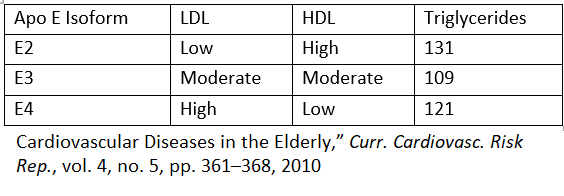
APOE4 as a risk factor for the development of Alzheimer’s disease
Every individual inherits one APOE gene variant from each parent. The likelihood of inheriting one or two APOE4 gene variants in the United States is 5% and 18% respectively. Alzheimer’s symptoms typically emerge around age 60 [37].
At age 70, individuals who carry one or more copies of the APOE4 gene are about three times more likely to develop Alzheimer’s disease than the average person, who typically carries two copies of the APOE3 gene [37].
Furthermore, APOE4 carriers are nearly seven times more likely to develop Alzheimer’s by age 70 than individuals carrying two copies of the APOE2 gene, which protects its fortunate carriers from developing Alzheimer’s disease [37].
ApoE2, ApoE3, and ApoE4 are associated with lower, average, and higher risks of disease development, respectively. This pie chart shows the distribution of ApoE genes in the population [10].
Alzheimer’s also compromises longevity
In addition to the quality-of-life reductions that Alzheimer’s patients experience, the median survival for Alzheimer’s patients ranges from 8.3 years for people diagnosed at age 65 to 3.4 years for people diagnosed at age 90 [38].
People who are diagnosed with Alzheimer’s disease at age 65 have a 67 percent reduction in lifespan compared to those without Alzheimer’s disease, while persons diagnosed at age 90 could anticipate a 39 percent reduction in lifespan [38]. Alzheimer’s disease causes more proportionate lifespan loss among younger than older sufferers [9].
Centenarian research shows that ApoE2 is protective against Alzheimer’s and cardiovascular disease. Furthermore, ApoE2 is associated with longevity independent of the reduction in Alzheimer’s risk it carries [9].
APOE4 as an independent risk factor for reduced longevity
Carriers of the APOE4 gene may have a significantly reduced life expectancy in the United States, Europe, and Asia. Carriers have reduced life expectancies, and this is not entirely due to Alzheimer’s disease [9].
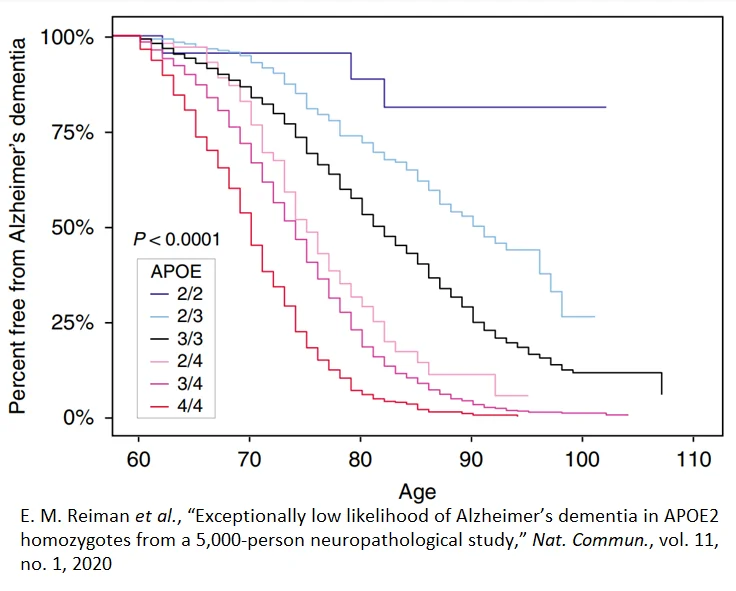
Among people 60 to 110 years old with two copies of the ApoE2 gene, the incidence and development of Alzheimer’s disease is very low compared to the remainder of the population. The percent free from Alzheimer’s dementia over 100 who carry the E2/E2 genotype is approximately 90%. On the other end of the spectrum, nearly 100% of E4/E4 genotypes at age 90 have Alzheimer’s disease. This suggests that therapeutic approaches that alter ApoE4 expression could hold a great deal of promise for the treatment of Alzheimer’s in the future.
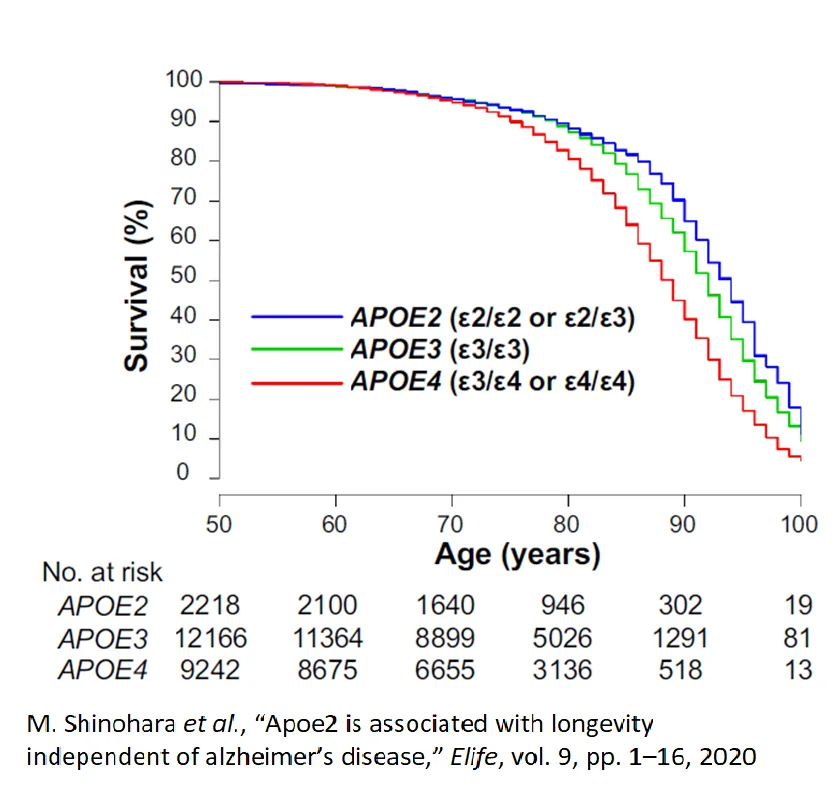
A study of 24,000 people adjusted for sex, age, race, and Alzheimer’s disease looked at longevity. Researchers assigned individuals to one of three groups: E2/E2 or E2/E3 in blue, E3/E3 in green, or E3/E4 or E4/E4 in red. The number of individuals surviving in the E2 group at age 90 was 50% greater than the number of E4 survivors at the same age. At age 100, survival for E2s is roughly double that of E4s [9].
This finding led investigators to establish a mouse model of Alzheimer’s to determine how the presence of different APOE gene variants might impact mouse behaviors related to longevity. Since mice don’t carry the same three gene variants for ApoE that humans do, the investigators added these genes to the mouse genome. Mice with the APOE2 gene lived for an average of 911 days, mice with the APOE3 gene lived 825 days, and mice with the APOE4 gene lived 753 days. The average APOE2 mouse lifespan was 21% longer than the average APOE4 mouse lifespan.
Researchers looked at the mobility of the mice and how often they stood on their hindquarters to quantify their activity. APOE2 mice were significantly more active than the APOE3 or APOE4 mice and spent considerably more time standing on their hindquarters, which requires more energy [39].
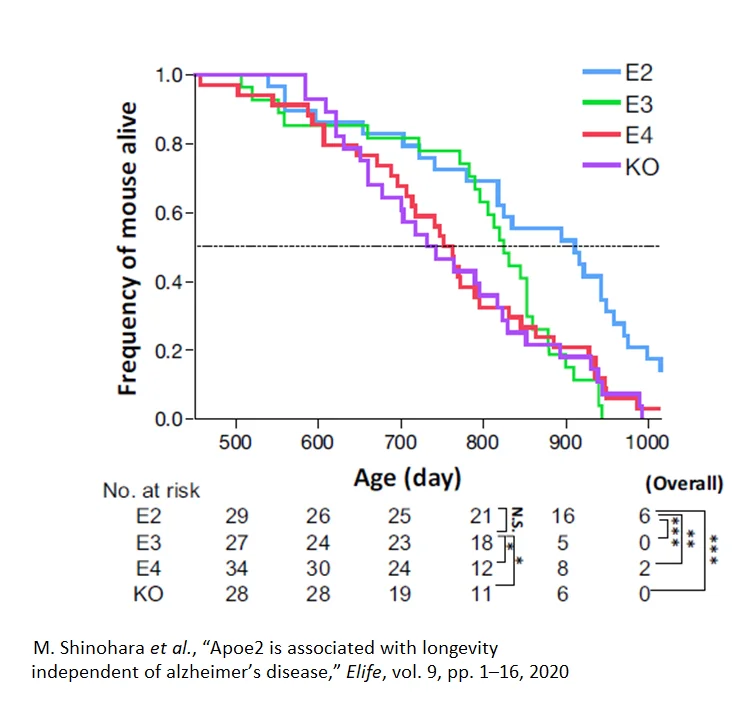
Furthermore, when the investigators looked at the blood lipids of the mice, they found the same pattern as in humans: E2 variant mice had relatively lower total cholesterol, higher HDL, and higher triglycerides than the other ApoE variants [9].
It is unclear if the longevity of the E2 mice is a function of their increased activity and lower cholesterol or if those characteristics simply correlate with E2 in mice. Numerous studies have shown that activity and cholesterol levels are associated with longevity [40-42].
Frequency of APOE4 and Alzheimer’s
5% of the population has two copies of the APOE4 gene, and 18% to 25% of the population has one copy of the APOE4 gene [42-44].
At age 70, individuals carrying one or more copies of the APOE4 gene are three times more likely to develop Alzheimer’s than the average individual. APOE4 carriers are nearly seven times more likely to develop Alzheimer’s than individuals carrying two copies of the APOE2 gene [9].
Several other genes influence the development of Alzheimer’s, including TREM2, CLU, CR1, CD33, ABCA7, APOC1, and TOMM40. In addition, there are variations in DNA sequences that make up the enhancer regions around APOE. Enhancer regions enhance the likelihood of nearby genes being expressed [45].
Sleep
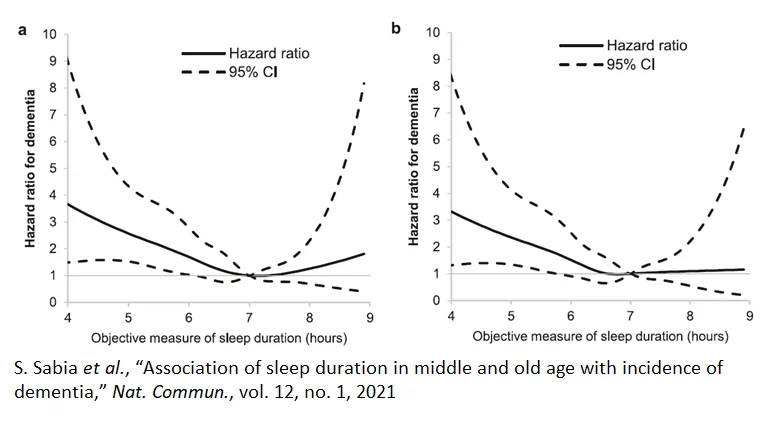
It is not clear if poor sleep causes dementia or is an early symptom of it. Nonetheless, evidence suggests that sleeping less than six hours a night or more than eight carries a 20-40% increase in the risk of developing dementia relative to sleeping about seven hours a night [46]. Poor sleep is also associated with the development of numerous diseases such as diabetes, which is a risk factor for developing Alzheimer’s [46-48].
Diet
There is sufficient data to recommend the Mediterranean [49] and MIND [50] diets to delay the onset of Alzheimer’s disease. The Mediterranean diet, the MIND diet (which includes elements designed to lower blood pressure), and other healthy eating patterns have been associated with cognitive benefits.
Research has shown that curcumin, an early diagnostic probe for amyloid beta, can prevent and protect against the advancement of Alzheimer’s disease. Curcumin effectively maintains the normal structure and function of cerebral vessels, mitochondria, and synapses, reducing risk factors for a variety of chronic diseases. The effect of curcumin on Alzheimer’s disease involves multiple signaling pathways: anti-amyloid and metal iron chelating properties, antioxidation, and anti-inflammatory activities [51,52].
Randomized, controlled trials of other vitamins and supplements have, in general, not shown statistically significant or consistent results [53,54]. However, a three-year study of 2,200 participants released in 2022 reports that a daily multivitamin-mineral supplement resulted in a statistically significant cognitive benefit. Improvements in cognition, however, should not be taken to mean that multivitamins slow the rate of amyloid beta accumulation, restore the blood-brain barrier, or bring about other organic changes that represent a slowing or reversal of Alzheimer’s disease progress [55].
Exercise
Regular physical activity benefits the brain. People who are physically active are less likely to experience a decline in mental function and have a reduced risk of developing Alzheimer’s disease. Physical activity level is an established modifiable risk factor for dementia in general and Alzheimer’s in particular. Plus, regular exercise helps combat other Alzheimer’s disease risk factors, such as depression, high blood pressure, diabetes, and obesity [56,57].
Exercising several times a week for 30 to 60 minutes may help maintain mental sharpness, improve memory, reasoning, judgment, and thinking skills for people with mild Alzheimer’s disease. Exercise delays the start of Alzheimer’s for people at risk of developing the disease or slows it progress. Lastly, exercise has been shown to increase the size of the hippocampus, a region of the brain associated with memory formation [56].
Relaxation
A 2015 review found that meditation, specifically Kirtan Kriya (KK), practiced 12 minutes a day, improves memory in studies of people with subjective cognitive decline, and mild cognitive impairment, which are two groups known to be at increased risk for the development of Alzheimer’s disease. One study has shown that KK improves sleep, decreases depression, reduces anxiety, down regulates inflammatory genes, upregulates immune system genes, improves insulin and glucose regulatory genes, and increases telomerase by 43%, the largest ever recorded [58].
Social health
According to the CDC, social isolation increases all-cause mortality and is associated with a near 50% increased risk of dementia. Therefore, having a strong social support network carries a significant reduction in the risk of Alzheimer’s disease development [59].
Literature
[1] CDC, “What is Alzheimer’s Disease? | CDC.”
[2] P. Huebbe and G. Rimbach, “Evolution of human apolipoprotein E (APOE) isoforms: Gene structure, protein function and interaction with dietary factors,” Ageing Res. Rev., vol. 37, pp. 146–161, 2017
[3] C. Fitzmaurice, “Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015,” JAMA Oncol., 2016
[4] K. P. Pahl, “Life expectancy in ancient and modern man,” Acta Anthropogenet., vol. 5, no. 2, pp. 119–128, 1981
[5] H. McDowell and A. A. Volk, “Infant Mortality BT – Evolutionary Perspectives on Infancy,” S. L. Hart and D. F. Bjorklund, Eds. Cham: Springer International Publishing, 2022
[6] R. Caspari and S.-H. Lee, “Is human longevity a consequence of cultural change or modern biology?,” Am. J. Phys. Anthropol., vol. 129, no. 4, pp. 512–517, Apr. 2006
[7] W. J. Strittmatter et al., “Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease.,” Proc. Natl. Acad. Sci., vol. 90, no. 17, pp. 8098–8102, Sep. 1993
[8] M. N. Haan and E. R. Mayeda, “Apolipoprotein E Genotype and Cardiovascular Diseases in the Elderly,” Curr. Cardiovasc. Risk Rep., vol. 4, no. 5, pp. 361–368, 2010
[9] M. Shinohara et al., “Apoe2 is associated with longevity independent of alzheimer’s disease,” Elife, vol. 9, pp. 1–16, 2020
[10] D. M. Hatters, C. A. Peters-Libeu, and K. H. Weisgraber, “Apolipoprotein E structure: insights into function,” Trends Biochem. Sci., vol. 31, no. 8, pp. 445–454, 2006
[11] A.-C. Raulin, Y. A. Martens, and G. Bu, “Lipoproteins in the Central Nervous System: From Biology to Pathobiology,” Annu. Rev. Biochem., vol. 91, no. 1, pp. 731–759, Jun. 2022
[12] J. D. Morrisett, R. L. Jackson, and A. M. Gotto, “Lipoproteins: Structure and Function,” Annu. Rev. Biochem., vol. 44, no. 1, pp. 183–207, Jun. 1975
[13] R. L. Raffaï, L.-M. Dong, R. V Farese, and K. H. Weisgraber, “Introduction of human apolipoprotein E4 ‘domain interaction’ into mouse apolipoprotein E,” Proc. Natl. Acad. Sci., vol. 98, no. 20, pp. 11587–11591, Sep. 2001
[14] K. R. Feingold, “Lipid and Lipoprotein Metabolism,” Endocrinol. Metab. Clin. North Am., vol. 51, no. 3, pp. 437–458, 2022
[15] G. F. Chen et al., “Amyloid beta: Structure, biology and structure-based therapeutic development,” Acta Pharmacol. Sin., vol. 38, no. 9, pp. 1205–1235, 2017
[16] T. Kanekiyo, H. Xu, and G. Bu, “ApoE and AB in Alzheimer’s disease: accidental encounters or partners?,” Neuron, vol. 81, no. 4, pp. 750–754, 2014
[17] K. Winkler et al., “Competition of AB; amyloid peptide and apolipoprotein E for receptor-mediated endocytosis,” J. Lipid Res., vol. 40, no. 3, pp. 447–455, Mar. 1999
[18] D. K. Strickland, S. L. Gonias, and W. S. Argraves, “Diverse roles for the LDL receptor family,” Trends Endocrinol. Metab., vol. 13, no. 2, pp. 66–74, 2002
[19] C. Frieden, H. Wang, and C. M. W. Ho, “A mechanism for lipid binding to apoE and the role of intrinsically disordered regions coupled to domain–domain interactions,” Proc. Natl. Acad. Sci., vol. 114, no. 24, pp. 6292–6297, Jun. 2017
[20] J. Kim et al., “Overexpression of Low-Density Lipoprotein Receptor in the Brain Markedly Inhibits Amyloid Deposition and Increases Extracellular AB Clearance,” Neuron, vol. 64, no. 5, pp. 632–644, Dec. 2009
[21] R. W. Mahley, K. H. Weisgraber, and Y. Huang, “Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS,” J. Lipid Res., vol. 50, no. SUPPL., pp. 183–188, 2009
[22] C. L. Maarouf, J. E. Walker, L. I. Sue, B. N. Dugger, T. G. Beach, and G. E. Serrano, “Impaired hepatic amyloid-beta degradation in Alzheimer’s disease,” PLoS One, vol. 13, no. 9, p. e0203659, Sep. 2018
[23] C. G. Fernandez, M. E. Hamby, M. L. McReynolds, and W. J. Ray, “The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer’s Disease ,” Frontiers in Aging Neuroscience , vol. 11. 2019
[24] C. Cruchaga et al., “Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease,” Hum. Mol. Genet., vol. 21, no. 20, pp. 4558–4571, 2012
[25] M. Narita, D. M. Holtzman, A. L. Schwartz, and G. Bu, “α2-Macroglobulin Complexes with and Mediates the Endocytosis of β-Amyloid Peptide via Cell Surface Low-Density Lipoprotein Receptor-Related Protein,” J. Neurochem., vol. 69, no. 5, pp. 1904–1911, Nov. 1997
[26] R. Deane, D. R. Bell, A. Sagare, and V. B. Zlokovic, “Clearance of Amyloid Beta Peptide Across the Blood-Brain Barrier: Implication for Therapies in Alzheimers Disease,” CNS & Neurological Disorders – Drug Targets, vol. 8, no. 1. pp. 16–30, 2009
[27] R. D. Bell et al., “SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells.,” Nat. Cell Biol., vol. 11, no. 2, pp. 143–153, 2009
[28] H. Saito et al., “Characterization of the Heparin Binding Sites in Human Apolipoprotein E,” J. Biol. Chem., vol. 278, no. 17, pp. 14782–14787, Apr. 2003
[29] M. Futamura, P. Dhanasekaran, T. Handa, M. C. Phillips, S. Lund-Katz, and H. Saito, “Two-step Mechanism of Binding of Apolipoprotein E to Heparin: Implication for the kinetics of apolipoprotein E-Heparan sulfate proteoglycan complex formation on cell surfaces,” J. Biol. Chem., vol. 280, no. 7, pp. 5414–5422, Feb. 2005
[30] W. J. S. de Villiers, D. R. van der Westhuyzen, G. A. Coetzee, H. E. Henderson, and A. D. Marais, “The Apolipoprotein E2(Arg145Cys) Mutation Causes Autosomal Dominant Type III Hyperlipoproteinemia With Incomplete Penetrance,” Arterioscler. Thromb. Vasc. Biol., vol. 17, no. 5, pp. 865–872, May 1997
[31] NORD, “Hyperlipoproteinemia Type III – NORD (National Organization for Rare Disorders).”
[32] L. Bergamaschini et al., “Peripheral Treatment with Enoxaparin, a Low Molecular Weight Heparin, Reduces Plaques and β-Amyloid Accumulation in a Mouse Model of Alzheimer’s Disease,” J. Neurosci., vol. 24, no. 17, pp. 4181–4186, 2004
[33] M. Xu et al., “Apolipoprotein e Gene Variants and Risk of Coronary Heart Disease: A Meta-Analysis,” Biomed Res. Int., vol. 2016, 2016
[34] B. A. Ference et al., “Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel,” Eur. Heart J., vol. 38, no. 32, pp. 2459–2472, Aug. 2017
[35] P. Barter, “The role of HDL-cholesterol in preventing atherosclerotic disease,” Eur. Hear. J. Suppl., vol. 7, no. suppl_F, pp. F4–F8, Jul. 2005
[36] M. R. Irvin et al., “Apolipoprotein E Polymorphisms and Postprandial Triglyceridemia Before and After Fenofibrate Treatment in the Genetics of Lipid Lowering and Diet Network (GOLDN) Study,” Circ. Cardiovasc. Genet., vol. 3, no. 5, pp. 462–467, Oct. 2010
[37] M. E. Belloy, V. Napolioni, and M. D. Greicius, “A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward,” Neuron, vol. 101, no. 5, pp. 820–838, 2019
[38] R. Brookmeyer, M. M. Corrada, F. C. Curriero, and C. Kawas, “Survival following a diagnosis of Alzheimer disease,” Arch. Neurol., vol. 59, no. 11, pp. 1764–1767, 2002
[39] K. Shinohara, E. ‐H. Kim, H. Omura, M. Fujimaki, M. Namiki, and H. Kato, Amino‐Carbonyl Reactions in Food and Biological Systems, 1986.
[40] S.-W. Yi, J.-J. Yi, and H. Ohrr, “Total cholesterol and all-cause mortality by sex and age: a prospective cohort study among 12.8 million adults,” Sci. Rep., vol. 9, no. 1, p. 1596, 2019
[41] M.-H. Kim et al., “Impact of Physical Activity on All-Cause Mortality According to Specific Cardiovascular Disease ,” Frontiers in Cardiovascular Medicine , vol. 9. 2022
[42] Y. Yamazaki, N. Zhao, T. R. Caulfield, C. C. Liu, and G. Bu, “Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies,” Nat. Rev. Neurol., vol. 15, no. 9, pp. 501–518, 2019
[43] NIH, “Alzheimer’s Disease Genetics Fact Sheet | National Institute on Aging.”
[44] M. M. Bos, R. Noordam, G. J. Blauw, P. E. Slagboom, P. C. N. Rensen, and D. Van Heemst, “The ApoE ϵ4 isoform: Can the risk of diseases be reduced by environmental factors?,” Journals Gerontol. – Ser. A Biol. Sci. Med. Sci., vol. 74, no. 1, pp. 99–107, 2019
[45] L. M. Bekris, F. Lutz, and C. E. Yu, “Functional analysis of APOE locus genetic variation implicates regional enhancers in the regulation of both TOMM40 and APOE,” J. Hum. Genet., vol. 57, no. 1, pp. 18–25, 2012
[46] S. Sabia et al., “Association of sleep duration in middle and old age with incidence of dementia,” Nat. Commun., vol. 12, no. 1, 2021
[47] M. Bellesi, L. de Vivo, M. Chini, F. Gilli, G. Tononi, and C. Cirelli, “Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex,” J. Neurosci., vol. 37, no. 21, pp. 5263–5273, May 2017
[48] R. Robbins, S. F. Quan, M. D. Weaver, G. Bormes, L. K. Barger, and C. A. Czeisler, “Examining sleep deficiency and disturbance and their risk for incident dementia and all-cause mortality in older adults across 5 years in the United States,” Aging (Albany. NY)., vol. 13, no. 3, pp. 3254–3268, 2021, doi: 10.18632/aging.202591.
[49] N. Scarmeas, Y. Stern, M. Tang, and J. A. Luchsinger, “Mediterranean Diet and Risk for Alzheimer’s Disease Nikolaos,” Ann. Neurol., vol. 59, no. 6, pp. 912–921, 2011
[50] D. A. Bennett and N. T. Aggarwal, “MIND diet associated with reduced incidence of Alzheimer’s disease.,” vol. 11, no. 9, pp. 1007–1014, 2016
[51] S. Abrahams, W. L. Haylett, G. Johnson, J. A. Carr, and S. Bardien, “Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review,” Neuroscience, vol. 406, pp. 1–21, 2019
[52] M. Chen, Z. Y. Du, X. Zheng, D. L. Li, R. P. Zhou, and K. Zhang, “Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease,” Neural Regen. Res., vol. 13, no. 4, pp. 742–752, 2018, doi: 10.4103/1673-5374.230303.
[53] A. Mielech, A. Puścion-Jakubik, R. Markiewicz-żukowska, and K. Socha, “Vitamins in alzheimer’s disease—review of the latest reports,” Nutrients, vol. 12, no. 11, pp. 1–15, 2020.
[54] NIH, “What Do We Know About Diet and Prevention of Alzheimer’s Disease?,” NIH. pp. 1–10, 2023
[55] L. D. Baker et al., “Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial,” Alzheimer’s Dement., vol. n/a, no. n/a, Sep. 2022
[56] Q. Meng, M. S. Lin, and I. S. Tzeng, “Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review,” Front. Neurosci., vol. 14, no. March, pp. 1–6, 2020
[57] M. Zhao, S. P. Veeranki, C. G. Magnussen, and B. Xi, “Recommended physical activity and all cause and cause specific mortality in US adults: Prospective cohort study,” BMJ, vol. 370, pp. 1–10, 2020.
[58] D. S. Khalsa, “Stress, meditation, and Alzheimer’s disease prevention: Where the evidence stands,” J. Alzheimer’s Dis., vol. 48, no. 1, pp. 1–12, 2015
[59] CDC, “Social Determinants of Health and Alzheimer’s Disease and Related Dementias.”



