Rejuvenation Roundup December 2021
2021 has come to a close, but rejuvenation biotechnology is still just beginning. Let’s see what the field has accomplished in December.
LEAF News
 Holiday Wishes from Lifespan.io: Lifespan.io president Keith Comito thanks the community of Lifespan.io for helping to bring an end to the diseases of aging, closes out the year, and looks forward to our further growth as a non-profit organization.
Holiday Wishes from Lifespan.io: Lifespan.io president Keith Comito thanks the community of Lifespan.io for helping to bring an end to the diseases of aging, closes out the year, and looks forward to our further growth as a non-profit organization.
EARD2021
Tim Maupin on Art and Longevity: Elena Milova of Lifespan.io interviewed Tim Maupin, an NYC-based filmmaker, about the way art can help change the way society views longevity research. As Elena pointed out, before we can see large-scale implementation of rejuvenation biotechnology, we have to convince people that greatly extended lifespans are good.
Tina Woods on a Longevity Ecosystem: Elena Milova interviewed Tina Woods on the development of a longevity ecosystem, the importance of language, and the social aspects of extending lifespans.
Lifespan News
Ageless Interview: Ryan O’Shea discusses our interview with Andrew Steele, an Oxford-trained physicist turned longevity-focused computational biologist, and the contents of his book, Ageless.
Journal Club
Telomere dysfunction, aging, and susceptibility to COVID-19: We talk about a study showing how age-related telomere dysfunction due to telomeric shortening or damage triggers DDR activation, which, in turn, causes the upregulation of ACE2, the SARS-CoV-2 cell receptor. This research paper outlines one of the reasons why elderly people are more susceptible to the ongoing COVID-19 pandemic.
Rejuvenation Roundup Podcast
Ryan O’Shea of Future Grind hosts this month’s podcast, showcasing the events and research discussed here.
Education
 What Is Quercetin? A Summary of Quercetin: Found in many fruits and vegetables, quercetin may have some potential in the context of aging. Here, we take a look at this natural antioxidant and popular dietary supplement.
What Is Quercetin? A Summary of Quercetin: Found in many fruits and vegetables, quercetin may have some potential in the context of aging. Here, we take a look at this natural antioxidant and popular dietary supplement.
Advocacy and Analysis
Separating Fact from Fiction in Anti-Aging Diets: Dr. Matt Kaeberlein and colleagues recently published a review summarizing anti-aging diets as well as their misconceptions. While fasting diets have been practiced for centuries, a recent re-emergence of this research has come to the anti-aging field.
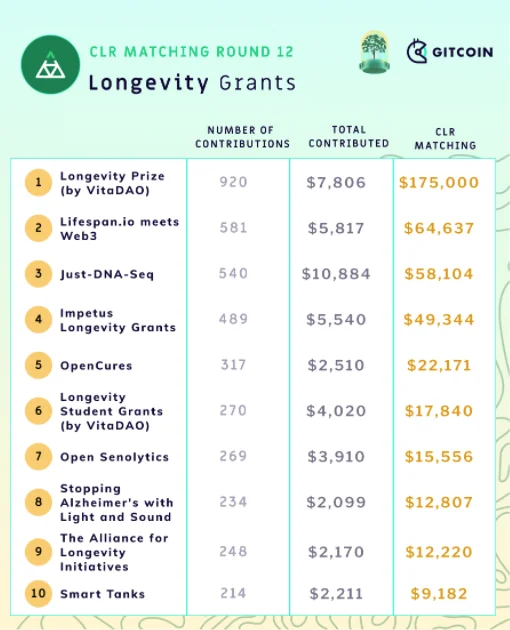
 Gitcoin GR12 Is a Win-Win for Aging Research: In recent years, the cryptocurrency community has become increasingly interested in the drive to increase healthy human lifespans. Gitcoin introduces longevity research into its funding rounds Gitcoin recently held its 12th funding round, and the community has chosen to raise cryptocurrency donations for aging research.
Gitcoin GR12 Is a Win-Win for Aging Research: In recent years, the cryptocurrency community has become increasingly interested in the drive to increase healthy human lifespans. Gitcoin introduces longevity research into its funding rounds Gitcoin recently held its 12th funding round, and the community has chosen to raise cryptocurrency donations for aging research.
Research Roundup
Bifidobacterium Adolescentis Improves Lifespan in Animals: A new publication in Nature Aging has investigated the role of the gut bacteria Bifidobacterium adolescentis in aging and longevity.
 Narrowed Neck Arteries Lead to Reduced Blood Flow in Eyes: Researchers publishing in GeroScience have found that carotid artery stenosis, a consequence of hypertension and a dangerous condition that is strongly associated with stroke, is linked to a lack of blood flow in the eyes.
Narrowed Neck Arteries Lead to Reduced Blood Flow in Eyes: Researchers publishing in GeroScience have found that carotid artery stenosis, a consequence of hypertension and a dangerous condition that is strongly associated with stroke, is linked to a lack of blood flow in the eyes.
Alpha-Ketoglutarate Decreases Biological Age in Human Study: A commercially available alpha-ketoglutarate-based supplement has been shown to roll back biological age by 7 years on average in a retrospective human study. Though metformin and rapamycin are getting most of the attention in the longevity field today, alpha-ketoglutarate (AKG) has emerged as another candidate longevity drug.
 Building a Universal Immunotherapy for Cancer: Researchers publishing in Cell Reports Medicine have described a method of using off-the-shelf, allogeneic immune cells as cancer therapy without the usual immunological risks associated with such cells.
Building a Universal Immunotherapy for Cancer: Researchers publishing in Cell Reports Medicine have described a method of using off-the-shelf, allogeneic immune cells as cancer therapy without the usual immunological risks associated with such cells.
Late-Life Treatment Extends Nematode Lifespan by Two-Fold: Scientists have managed to extend the lifespan of C. elegans nematode worms by as much as 135% by blocking an insulin-related pathway very late in life. Back in the late 80s and early 90s, experiments with C. elegans, tiny nematode worms, became one of geroscience’s first major successes, but this recent study uses a different approach.
 Hyperbaric Oxygen Causes Transcriptomic Changes: Transcriptomic results have been published regarding the Shamir Medical Center longitudinal study on hyperbaric oxygen therapy (HBOT), which was conducted between 2016 and 2020 on 35 healthy adults aged 64 and older.
Hyperbaric Oxygen Causes Transcriptomic Changes: Transcriptomic results have been published regarding the Shamir Medical Center longitudinal study on hyperbaric oxygen therapy (HBOT), which was conducted between 2016 and 2020 on 35 healthy adults aged 64 and older.
A Case Study Shows How Cord Blood Ameliorates Progeria: A new case report published in the International Journal of Molecular Sciences highlights cardiovascular improvements in a patient with Hutchinson-Gilford progeria treated with umbilical cord blood. Hutchinson-Gilford Progeria SyndromeHutchinson-Gilford progeria syndrome, also known simply as progeria, is a disease characterized by premature aging.
 A Licorice Derivative Restores Adipose Stem Cells in Vitro: Researchers publishing in Aging have reported that licochalcone A (LA), a flavonoid that originates from Chinese licorice, restores human adipose-derived stem cells (hADSCs) in cell culture. Previous research on Licochalcone A has discovered that it has anti-inflammatory and anti-tumor effects, reduces obesity in mice, and protects against liver failure.
A Licorice Derivative Restores Adipose Stem Cells in Vitro: Researchers publishing in Aging have reported that licochalcone A (LA), a flavonoid that originates from Chinese licorice, restores human adipose-derived stem cells (hADSCs) in cell culture. Previous research on Licochalcone A has discovered that it has anti-inflammatory and anti-tumor effects, reduces obesity in mice, and protects against liver failure.
Viagra Identified as a Candidate Drug for Alzheimer’s: After sifting through 1,600 FDA-approved drugs, scientists have shown that Viagra is significantly correlated with lower Alzheimer’s disease risk. Viagra (sildenafil) is one of the most recognizable drugs in the world, but originally, sildenafil was studied as a heart drug.
 Olive Derivative Fights Epigenetic Kidney Aging: Researchers publishing in Aging Cell have discovered how and why oleuropein (OLP), a polyphenol derived from olives, ameliorates epigenetic kidney aging.
Olive Derivative Fights Epigenetic Kidney Aging: Researchers publishing in Aging Cell have discovered how and why oleuropein (OLP), a polyphenol derived from olives, ameliorates epigenetic kidney aging.
A New Plant-Derived Senomorphic Slows Aging in Mice: Studying grape seed extract, scientists have discovered a new senomorphic compound that enhances chemotherapy and prolongs lifespan and healthspan in mice.
 Epigenetic Clock Shows Association With Cardiovascular Aging: A study published in Mechanisms of Ageing and Development has found correlations between cardiovascular aging and a measurement of epigenetic age acceleration. It suggests that some components of cardiovascular disease may be more related to measures of epigenetic age acceleration than others.
Epigenetic Clock Shows Association With Cardiovascular Aging: A study published in Mechanisms of Ageing and Development has found correlations between cardiovascular aging and a measurement of epigenetic age acceleration. It suggests that some components of cardiovascular disease may be more related to measures of epigenetic age acceleration than others.
A Plant-Based Diet Is Correlated With a Less Leaky Gut: The MaPLE trial in Italy investigated whether or not increasing fruits and vegetables decreases leaky gut syndrome. This study examines increased intestinal permeability in adults 60 years and older, as age has been reported to be a risk factor for increased intestinal permeability over the age of 50.
 Old Serum Shown to Kill Brain Stem Cells: A study published in Aging and Disease has shown how exposure to old serum causes hippocampal progenitor cells (HPCs), which form new neurons, to die. To begin their study, the researchers examined a middle-aged to old cohort ranging in age from 52 to 89.
Old Serum Shown to Kill Brain Stem Cells: A study published in Aging and Disease has shown how exposure to old serum causes hippocampal progenitor cells (HPCs), which form new neurons, to die. To begin their study, the researchers examined a middle-aged to old cohort ranging in age from 52 to 89.
A Vaccine for High Cholesterol Works in Mice: Researchers publishing in Cell Reports Medicine have discovered that a vaccine targeting angiopoietin-like protein 3 (ANGPTL3) alleviates dyslipidemia, a term for poor cholesterol levels, and improves outcomes in obese mice.
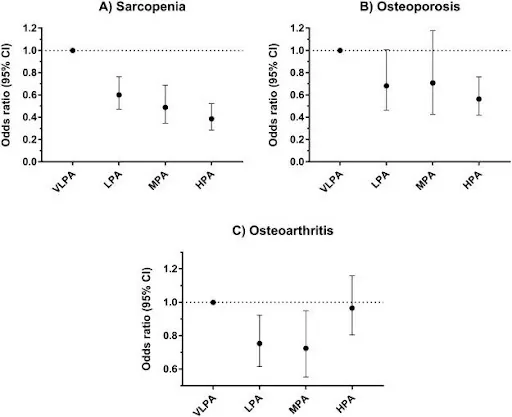
 The Effects of Physical Activity on Frailty: Sarcopenia, osteoporosis, and osteoarthritis are the trio of age-related diseases that affect, respectively, muscles, bones, and joints. This study shows that even low levels of physical activity are very good for all three, but exercising too much can potentially harm you.
The Effects of Physical Activity on Frailty: Sarcopenia, osteoporosis, and osteoarthritis are the trio of age-related diseases that affect, respectively, muscles, bones, and joints. This study shows that even low levels of physical activity are very good for all three, but exercising too much can potentially harm you.
Senolytics Improve Muscle Regeneration Only in Old Animals: A study published in Aging Cell has found divergent results between the post-injury muscle regeneration of young and old mice treated with senolytics. This study showed that infiltrating macrophages after injury become senescent in older mice and impair the healing process.
 A Potential Vaccine for Senescent Cells: The journal Nature has published a letter communication regarding a senolytic vaccination study. The researchers examined transcriptomic data and analyzed the expression profiles for two specific genes using the Gene Expression Omnibus Database.
A Potential Vaccine for Senescent Cells: The journal Nature has published a letter communication regarding a senolytic vaccination study. The researchers examined transcriptomic data and analyzed the expression profiles for two specific genes using the Gene Expression Omnibus Database.
NMN Slows Ovarian Aging in Mice: Scientists have found that long-term supplementation with NMN, an NAD+ precursor, dramatically slows reproductive aging in mice by improving mitochondrial function and alleviating senescence.
 A Link Between the Circadian Rhythm and Aging: A new study published in Aging has shown a relationship between the circadian clock, which governs our daily biological rhythms, and the changes in gene expression with age.
A Link Between the Circadian Rhythm and Aging: A new study published in Aging has shown a relationship between the circadian clock, which governs our daily biological rhythms, and the changes in gene expression with age.
Senescence-induced changes in CD4 T cell differentiation can be alleviated by treatment with senolytics: Treatment of aged mice with senolytics prior to influenza infection restored the differentiation of Th cells in those aged mice to a more youthful phenotype.
Evaluation of the Anti-Aging Effects of a Probiotic Combination Isolated From Centenarians in a SAMP8 Mouse Model: The anti-aging effects of the probiotic combination may be through the regulating intestinal microbiota and inhibiting TLR4/NF?B-induced inflammation. This research provides the basis and technical support for the future production and application of the probiotic combination.
Functional rejuvenation of aged neural stem cells by Plagl2 and anti-Dyrk1a activity: Aging of neural stem cells can be reversed to induce functional neurogenesis continuously, offering a way to treat age-related neurological disorders.
Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles: These vesicles play a key role in the rejuvenating effects of heterochronic blood exchange, and Klotho transcripts within them phenocopy the effects of young serum on aged skeletal muscle.
Exercise plasma boosts memory and dampens brain inflammation via clusterin: These findings demonstrate the existence of anti-inflammatory exercise factors that are transferrable, target the cerebrovasculature and benefit the brain, and are present in humans who engage in exercise.
Small molecules for cell reprogramming: a systems biology analysis: The researchers conclude that further investigation of small molecules and their relationship with longevity regulators will be helpful for developing optimal cocktails for cell reprogramming.
Epigenetic therapy attenuates oxidative stress in bone marrow-derived mesenchymal stem cells during aging: These findings revealed novel crosstalk between histone epigenetic modification and oxidative stress during stem cell aging, suggesting the possibility of epigenetic therapy in the recovery of BMSC senescence and treatment of age-related bone disease.
Profiling epigenetic age in single cells: The researchers use a new framework, scAge, to reveal a natural and stratified rejuvenation event occurring during early embryogenesis. They provide this framework as a resource to enable exploration of epigenetic aging trajectories at single-cell resolution.
DNA methylation clocks tick in naked mole rats but queens age more slowly than nonbreeders: Epigenetic clocks revealed that breeding queens age more slowly than nonbreeders, a feature that is also observed in some eusocial insects. These results show that despite a phenotype of negligible senescence, the naked mole rat ages epigenetically.
Spermidine supplementation influences mitochondrial number and morphology in the heart of aged mice: Mitochondria of the aged mouse left ventricle exhibited changes in number and 3D ultrastructure that is likely the structural correlate of dysfunctional mitochondrial dynamics. Spermidine treatment reduced, at least in part, these morphological changes, indicating a beneficial effect on cardiac mitochondrial alterations associated with aging.
Oligomer-Targeting Prevention of Neurodegenerative Dementia by Intranasal Rifampicin and Resveratrol Combination: These results show the advantages of this combinatorial medicine with regards to safety and effectiveness over single-drug rifampicin. The findings may provide a feasible means for the prevention of neurodegenerative dementia that targets toxic oligomers.
No limit to maximal lifespan in humans: how to beat a 122-year-old record: Well-known gerontologist Mikhail Blagosklonny shows that combining anti-aging medicine with cutting-edge medical care, regardless of chronological age, will extend maximal lifespan further.
News Nuggets
 Apollo Health Ventures Closes $180 Million Fund: Apollo Health Ventures, a venture capitalist firm focusing on rejuvenation biotechnology, has raised $180 million in order to fund companies developing interventions that target age-related diseases.
Apollo Health Ventures Closes $180 Million Fund: Apollo Health Ventures, a venture capitalist firm focusing on rejuvenation biotechnology, has raised $180 million in order to fund companies developing interventions that target age-related diseases.
Gitcoin Launches Crypto Funding Round for Longevity: Gitcoin is a platform dedicated to supporting the development of open-source Web3 software – decentralized architecture based upon blockchain technology – and the funding of “public goods” projects that are intended to benefit everyone. Of course, one such public good is the extension of healthy human lifespan, including our own projects.
 New Partnership Between Forever Healthy and Buck Institute: Yet another group has formed to advance early-stage biotechnology research. This time, the Forever Healthy Foundation, founded by Michael Greve, has formed a $5 million partnership with the Buck Institute in order to fund rejuvenation biotechnology.
New Partnership Between Forever Healthy and Buck Institute: Yet another group has formed to advance early-stage biotechnology research. This time, the Forever Healthy Foundation, founded by Michael Greve, has formed a $5 million partnership with the Buck Institute in order to fund rejuvenation biotechnology.
NewLimit: A New Biotech Working on Epigenetic Aging: Long gone are the times when longevity biotech companies were scarce and poorly funded. Two investors, including a co-founder of Coinbase, have announced a new biotech venture that aims to solve aging by studying its epigenetic aspects.
 Ichor Life Sciences Acquires Woodland Biosciences, Inc.: Woodland Biosciences, which studies metabolism and cancer, has joined the holdings of Ichor Life Sciences. Ichor intends to use Woodland’s expertise and research to increase the specializations of its pharmacological division.
Ichor Life Sciences Acquires Woodland Biosciences, Inc.: Woodland Biosciences, which studies metabolism and cancer, has joined the holdings of Ichor Life Sciences. Ichor intends to use Woodland’s expertise and research to increase the specializations of its pharmacological division.








 As we close out the year and continue the holiday season, I want to take this opportunity to thank you for being part of our community at Lifespan.io and helping to bring an end to the diseases of aging. 2021 has been another challenging year, but you did not let that stop you, and together, we have accomplished amazing things.
As we close out the year and continue the holiday season, I want to take this opportunity to thank you for being part of our community at Lifespan.io and helping to bring an end to the diseases of aging. 2021 has been another challenging year, but you did not let that stop you, and together, we have accomplished amazing things.