A New Startup Is Going After Rogue Cells
In a blog post, the founder and CEO of Arda Therapeutics, Adam Freund, explained his company’s intriguing strategy along with why it is imperative to counter aging.
From Calico to Arda
It’s not every day that the longevity field sees a new player with a fresh message joining the ranks. On January 25th, Dr. Freund announced the arrival of Arda Therapeutics in a blog post. After completing his Ph.D. in molecular and cell biology at Berkeley, Dr. Freund was briefly a postdoctoral fellow at the Buck Institute under Judith Campisi, and then at Stanford, working on projects that focused on aging and cancer biology. Importantly, before founding Arda, Dr. Freund spent almost seven years as a principal investigator at Calico. Freund spoke about his work at Calico at last year’s Longevity Therapeutics conference.
According to Freund, Arda Therapeutics is backed by some well-known venture capital funds, including Andreessen Horowitz and The Longevity Fund. The company is also actively hiring, so we do not yet know who will join Freund on the scientific team.
More than senolytics
Arda proposes an interesting strategy based on eliminating harmful cells. This is already being done for cancer and senescent cells, but Freund makes an argument for a much wider approach based on the fact that many diseases are caused by dysregulation of a relatively small subset/subtype of cells. As an example, he mentions overgrowth of stromal cells that can lead to fibrosis and overactivation of certain immune cell types that causes chronic inflammation [1].
The traditional approach is to bring those cells back to normal with drugs, but this can be very challenging because of the immense complexity and interconnectedness of cellular processes, many of which are still unknown to us.
Rather that trying to fix misbehaving cells, it might be easier to simply eliminate them. Scientists know a great deal about the mechanisms of cellular death and how to trigger them. At times, the body can repopulate the niche, replacing the dead cells with healthy ones.
For this approach to succeed, such cells must be precisely identified. This is not an easy task, but recent advances in bioscience and AI can provide a roadmap. In this regard, Freund mentions single-cell sequencing that allows researchers to build much more detailed cell maps based on subtle differences between cellular subtypes. Interestingly, from the few papers that have been published by Calico, we know that its scientists use large-scale single-cell sequencing [2].
In cancer, trying to eliminate harmful cells involves harsh treatments that exert a heavy toll on the patient’s health and quality of life. According to Freund, the problem is that cancer cells proliferate uncontrollably, which is not the case with other harmful cells. As a result, to eradicate cancer, every single cancer cell must be killed, while to alleviate other diseases, it might be enough to simply lower the burden of “bad cells”. We would add that due to their fast proliferation, cancer cells continuously evolve to evade threats, which makes them an even more formidable adversary.
In his blog post, Freund is being remarkably honest about the challenges that lie ahead. For instance, even when a harmful subtype of cells is identified, it might be tricky to devise a precise targeting and delivery method. Yet, recent breakthroughs in this field, such as lipid nanoparticles [3], inspire hope.
Unabashedly anti-aging
Freund does not shy away from admitting that his company will target age-related diseases and aging itself, as he explains his beef with aging in a passionate and eloquent way:
For as long as I have been a scientist, I have been driven to understand aging. Frankly, I have trouble understanding why it’s not a more common obsession. After all, it’s going to kill you… At Arda, we hypothesize that there are shared biological mechanisms that affect multiple aspects of age-related deterioration, and that targeting those mechanisms is a promising path to extending healthy lifespan. More specifically, we suspect that many aspects of aging are driven by the hyper-activation of particular cell types, and that if we can remove those cells, we will delay (and possibly reverse) tissue deterioration. If we are even marginally successful, we will push back the greatest killer that has ever existed, improve quality of life at older ages, and give everyone more time with the people they love. We strive to not just add years to life, but life to years.
We at Lifespan.io couldn’t agree more. Unfortunately, Freund’s attitude is in sharp contrast with some other companies, such as Altos Labs, that are trying to position themselves as strictly non-longevity, even though cellular reprogramming, the main avenue that Altos Labs will be pursuing, is more longevity-oriented than clearing out rogue cells.
Literature
[1] Thameem Dheen, S., Kaur, C., & Ling, E. A. (2007). Microglial activation and its implications in the brain diseases. Current medicinal chemistry, 14(11), 1189-1197.
[2] Roux, A., Zhang, C., Paw, J., Zavala-Solorio, J., Vijay, T., Kolumam, G., … & Kimmel, J. C. (2021). Partial reprogramming restores youthful gene expression through transient suppression of cell identity. bioRxiv.
[3] Li, Y., Su, Z., Zhao, W., Zhang, X., Momin, N., Zhang, C., … & Weiss, R. (2020). Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nature Cancer, 1(9), 882-893.











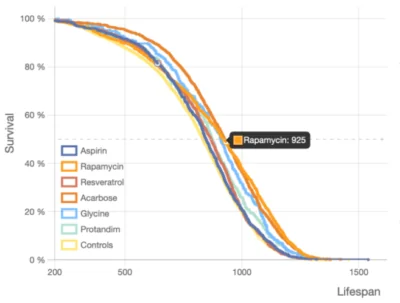 Take a close look at these so-called lifespan curves, each of which shows a surviving fraction of a cohort as a function of time, always going from 100% to zero. The precise shape of such a curve and slight differences between multiple curves tell us a lot about an intervention and its interaction with an organism.
Take a close look at these so-called lifespan curves, each of which shows a surviving fraction of a cohort as a function of time, always going from 100% to zero. The precise shape of such a curve and slight differences between multiple curves tell us a lot about an intervention and its interaction with an organism.