Antibody Therapy Alone Eliminates Certain Rectal Cancers
Researchers publishing in the New England Journal of Medicine have been able to completely eliminate stage 2 and 3 rectal tumors with a single monoclonal antibody drug [1].
Therapy comes with a price
If we leave out skin cancers, colorectal cancer is the third most commonly diagnosed cancer in the US. According to the American Cancer Society, in 2022, more than 106,000 Americans will get colon cancer and 46,000 will get rectal cancer.
On the brighter side, the prevalence of colorectal cancer has declined in recent decades, probably due to early detection and extraction of polyps before they can actually turn into tumors. Early diagnostics and better treatment have also significantly bolstered the survival rate.
However, immediate survival is not the end of the story. Chemo- and radiotherapy negatively affect patients’ quality of life and decrease their healthspan and lifespan, while rectum excision surgery is highly invasive and has harmful long-term consequences.
Evading detection
Immune therapy, including PD-1 blockade, has recently emerged as a supplementary treatment for certain cancers [2]. PD-1 (programmed cell death protein 1) is a receptor on immune cells that dampens their activity when it binds to a ligand (PD-L1 or PD-L2) on the surface of a non-immune cell. This mechanism prevents an autoimmune reaction, but it is often hijacked by tumor cells. By expressing the ligand, tumor cells fool immune cells into misrecognizing them as benign, law-abiding members of the cellular community.
Blocking PD-1 with monoclonal antibodies suppresses this mechanism and boosts anti-tumor immune response (although it also increases autoimmune reaction). Some cancer types are known to respond better to PD-1 blockade, including a subset of rectal cancer called mismatch-repair deficient rectal cancer. Mismatch repair is one of the mechanisms that cells use to repair DNA damage, and its deficiency leads to a higher rate of mutations in the cell. Interestingly, mismatch-repair deficient cancers are usually more resistant to chemo [3], which makes PD-1 blockade a weapon of choice against them.
Complete response
In this new phase 2 study, 16 patients were recruited, and 12 completed the treatment. 15 participants had stage 3 rectal cancer, and one had stage 2. At those stages, which represent the majority of cases, cancer is still mostly localized to the rectum and adjacent tissues, and the five-year survival rate is around 70%. Current protocols usually begin with chemo- and radiotherapy and then proceed to surgery. The participants received dostarlimab, a PD-1 blocking monoclonal antibody agent, intravenously every three weeks for six months.
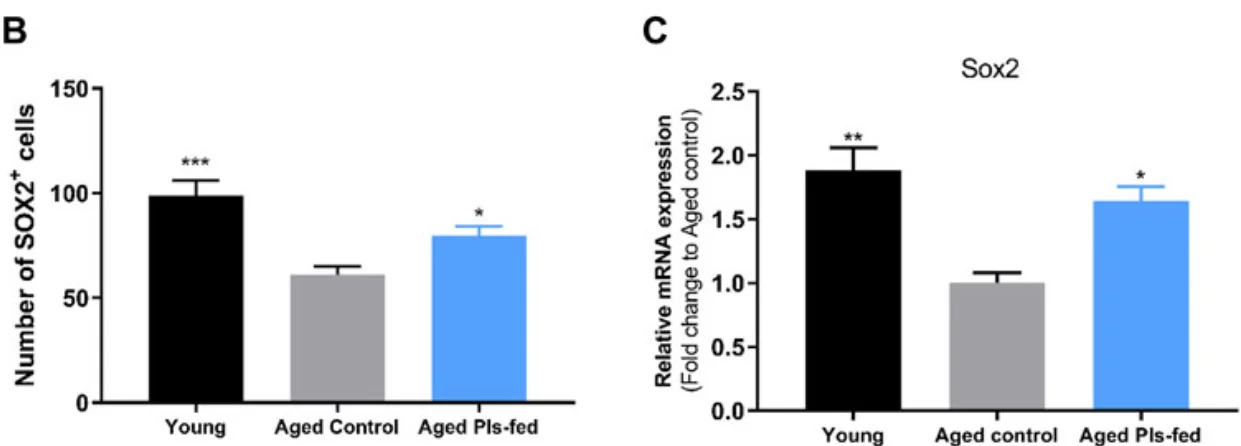
The researchers hoped that the drug would improve the participants’ chances, but they certainly were not ready for what was to come: after receiving the antibody treatment, all patients demonstrated so-called “clinical complete response”, which means that there was no evidence of tumor as measured by MRI, PET, endoscopic evaluation, digital rectal examination, or biopsy. According to the researchers, such wall-to-wall success is unprecedented in cancer research. Even six months later, none of the patients had required additional therapy. The well-known side effects of monoclonal antibody therapy were mostly mild.
Needs replication
As spectacular as those results are, usual caveats apply. First, the results will need to be replicated in a larger study. Second, only 5-10% of all colorectal cancers are mismatch-repair deficient. Third, the endpoint used (clinical complete response) does not equal total elimination of cancer, and the follow-up period was not nearly enough to conclude that all patients have become cancer-free. Finally, metastatic colorectal cancer remains a huge problem: while it now accounts for just 22% of all cases, the five-year survival rate is only 14%.
The researchers attribute their success to several factors, including the relatively long duration of the treatment. It is also obvious that for reasons largely unknown, this particular type of cancer is especially sensitive to PD-1 blockade. The authors also discuss the important question of why metastatic colorectal cancer responds to the same treatment poorly, despite having the same molecular characteristics. They arrive at an interesting conclusion that the gut microbiome augments the treatment’s efficacy, which would be in line with some previous research [4].
In our study, single-agent dostarlimab was remarkably effective in mismatch repair–deficient, locally advanced rectal cancer, providing a clinical complete response in all 12 patients who have completed treatment to date. The study also provides a framework for evaluation of highly active anticancer therapies in the neoadjuvant context, wherein patients would potentially be spared from chemoradiotherapy and surgery while their tumor is treated when it is most likely to respond — namely, before exposure to other agents that might select for cells with a resistant phenotype.
Conclusion
Immunotherapy for cancer is quickly becoming more effective and sophisticated. This study demonstrates for the first time that immunotherapy alone might be enough in some cases to suppress cancer, eliminating the need for more traditional therapies that can be taxing on patients.
Literature
[1] Cercek, A., Lumish, M., Sinopoli, J., Weiss, J., Shia, J., Lamendola-Essel, M., El Dika, I. H., Segal, N., Shcherba, M., Sugarman, R., Stadler, Z., Yaeger, R., Smith, J. J., Rousseau, B., Argiles, G., Patel, M., Desai, A., Saltz, L. B., Widmar, M., Iyer, K., … Diaz, L. A., Jr (2022). PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. The New England journal of medicine, 10.1056/NEJMoa2201445. Advance online publication.
[2] Kuchroo, J. R., Hafler, D. A., Sharpe, A. H., & Lucca, L. E. (2021). The double-edged sword: Harnessing PD-1 blockade in tumor and autoimmunity. Science Immunology, 6(65), eabf4034.
[3] Cercek, A., Fernandes, G. D. S., Roxburgh, C. S., Ganesh, K., Ng, S., Sanchez-Vega, F., … & Stadler, Z. K. (2020). Mismatch Repair–Deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clinical Cancer Research, 26(13), 3271-3279.
[4] Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A., Andrews, M. C., Karpinets, T. V., … & Wargo, J. (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science, 359(6371), 97-103.