Rejuvenation Roundup December 2022
The holiday season is over, the new year is upon us, and we return to our mission of giving us many more years to come. Here’s what’s been done on the rejuvenation front last month.
LEAF News
Team and activities
 Wishing You a Happy Holiday and a Healthy New Year!: Executive Director Stephanie Dainow and the rest of the Lifespan.io team wished everyone a healthy holiday season at the end of 2022.
Wishing You a Happy Holiday and a Healthy New Year!: Executive Director Stephanie Dainow and the rest of the Lifespan.io team wished everyone a healthy holiday season at the end of 2022.The Science-Packed Longevity Summit at the Buck Institute: The Longevity Summit at the Buck Institute, a two-day geroscience and longevity biotech conference held on December 6-7, was nevertheless densely packed with new research.
Lifespan News
Saving for Longevity: Ryan O’Shea talks about a recent study showing how people’s thoughts about saving money change when confronted with how long they might actually live. Many people retire only to realize that they haven’t saved enough money to be comfortable.
Rapamycin and Egg Cells: Ryan O’Shea discusses a recent study showing that rapamycin improves the potential of egg cells to form embryos. Egg cell viability declines much more rapidly than other aspects of aging accumulate, but there may be ways of improving it.
New Support for Longevity: Foundations that use crypto donations to fund longevity science are the topic of this Lifespan News episode. Both SENS Research Foundation and Lifespan.io have launched end-of-the-year campaigns to support groundbreaking work.
Rejuvenation Roundup Podcast
Ryan O’Shea of Future Grind hosts this month’s podcast, showcasing the events and research discussed here.
Journal Club
For December’s edition of Journal Club, Dr. Oliver Medvedik took a look at a new paper that explores phytocannabinoids in the context of skin aging and rejuvenation.
Advocacy and Analysis
 De-Aging Movie Stars Won’t Solve Aging: In the fifth installment of the popular Indiana Jones franchise, Harrison Ford will reportedly be “de-aged” by means of computer graphics, presumably to make him kicking Nazi butt at least somewhat believable. A regular person would react to this piece of showbiz gossip with a chuckle, but for a longevity enthusiast, this raises a bunch of important questions.
De-Aging Movie Stars Won’t Solve Aging: In the fifth installment of the popular Indiana Jones franchise, Harrison Ford will reportedly be “de-aged” by means of computer graphics, presumably to make him kicking Nazi butt at least somewhat believable. A regular person would react to this piece of showbiz gossip with a chuckle, but for a longevity enthusiast, this raises a bunch of important questions.
The Intersection of Crypto, Gamification, and Longevity: Gamification, the concept of using gaming elements in a non-traditional setting, has long been criticized as at best, a banal pastime, and at worst, a devious manipulation tool employed by unscrupulous companies to acquire profit. However, supporters suggest the opposite: that gamification is a science that utilizes a variety of well-established tools.
Research Roundup
 Polyphenols Help Reduce Visceral Fat: Pitching two variants of the Mediterranean diet against each other in a randomized controlled trial, scientists have found that the plant-oriented one, which contained more polyphenols, was more effective for weight loss.
Polyphenols Help Reduce Visceral Fat: Pitching two variants of the Mediterranean diet against each other in a randomized controlled trial, scientists have found that the plant-oriented one, which contained more polyphenols, was more effective for weight loss.
Rapamycin, not Dietary Restriction, Fights Infection in Mice: A meta-analysis published in GeroScience has shown that the well-studied drug rapamycin has positive effects on infection in mice, but dietary restriction seems to worsen the problem.
 Finding Interventions That Truly Impact Aging: Researchers publishing in Nature Communications have determined that interventions that extend lifespan in mice may not have significant effects on the processes of aging. Improvement is not the same as slowing decline.
Finding Interventions That Truly Impact Aging: Researchers publishing in Nature Communications have determined that interventions that extend lifespan in mice may not have significant effects on the processes of aging. Improvement is not the same as slowing decline.
Intermittent Fasting Protects Mice from Brain Injury: Scientists from Singapore have found that intermittent fasting alleviates damage incurred by chronic cerebral hypoperfusion, the impaired blood flow to the brain that is thought to be a cause of age-related dementia.
 Snowsports for Improved Balance: In a new study published in Journal of Science and Medicine in Sport (JSAMS) Plus, researchers have shown that participating in such sports as snowboarding and skiing might counteract age-associated proprioception decline.
Snowsports for Improved Balance: In a new study published in Journal of Science and Medicine in Sport (JSAMS) Plus, researchers have shown that participating in such sports as snowboarding and skiing might counteract age-associated proprioception decline.
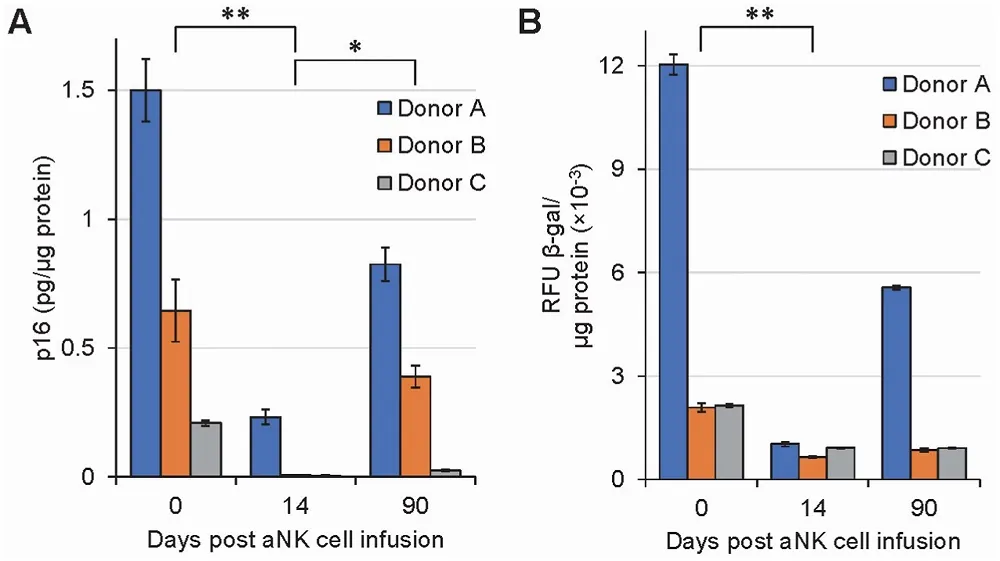
Activated Natural Killer Cells Fight Senescence in Humans: In a study recently published in Biochemistry and Biophysics Reports, researchers have shown that growing natural killer (NK) cells and re-introducing them back into the human bloodstream reduces senescence markers in a wide variety of immune cells.
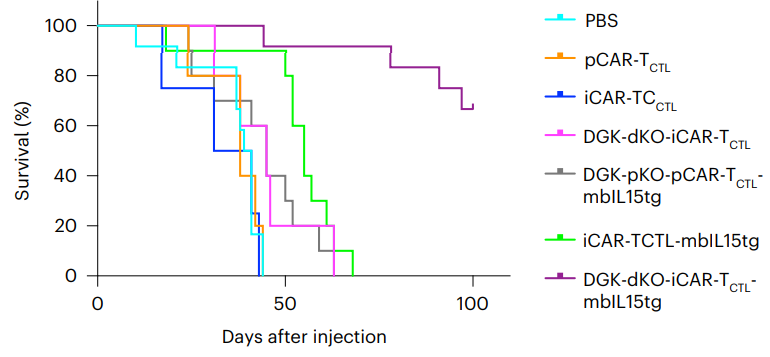
 Genetically Enhancing T Cells to Fight Tumors: A team of researchers from multiple Japanese universities has found a way to genetically enhance T cells against solid tumors, as published today in Nature Biomedical Engineering. A focus on signals This highly in-depth paper begins with a discussion of signaling in the response of chimeric antigen receptor (CAR) T cells.
Genetically Enhancing T Cells to Fight Tumors: A team of researchers from multiple Japanese universities has found a way to genetically enhance T cells against solid tumors, as published today in Nature Biomedical Engineering. A focus on signals This highly in-depth paper begins with a discussion of signaling in the response of chimeric antigen receptor (CAR) T cells.
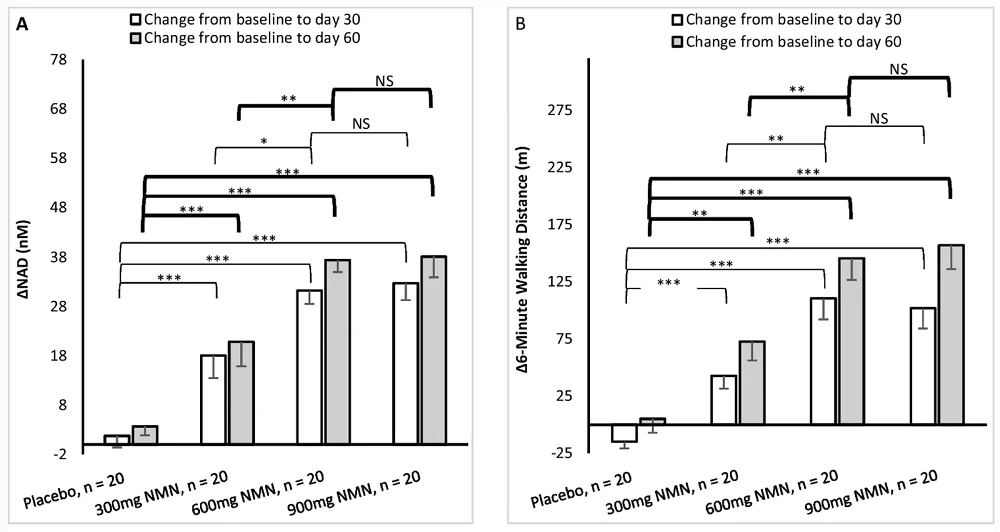
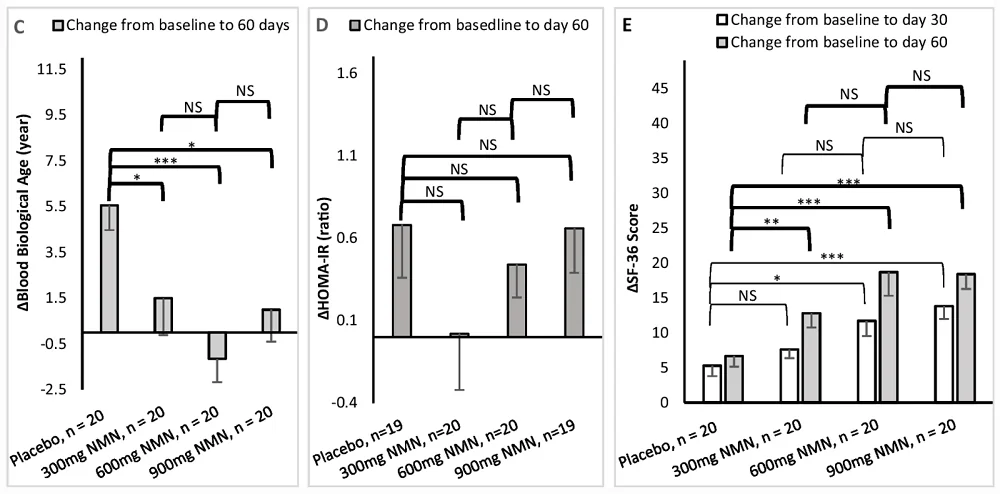
Study Suggests NMN May Improve NAD+ and Walking Speed: A new study suggests that NMN supplementation elevates NAD+ levels and increases walking distance in healthy participants, with 600 mg/day being the optimal dose. This study was based on a randomized, multicenter, double-blind, placebo-controlled clinical trial.
 Size Matters in Cellular Aging: In a new review article published in Frontiers in Cell and Developmental Biology, researchers have suggested adding cellular enlargement to the hallmarks of aging.
Size Matters in Cellular Aging: In a new review article published in Frontiers in Cell and Developmental Biology, researchers have suggested adding cellular enlargement to the hallmarks of aging.
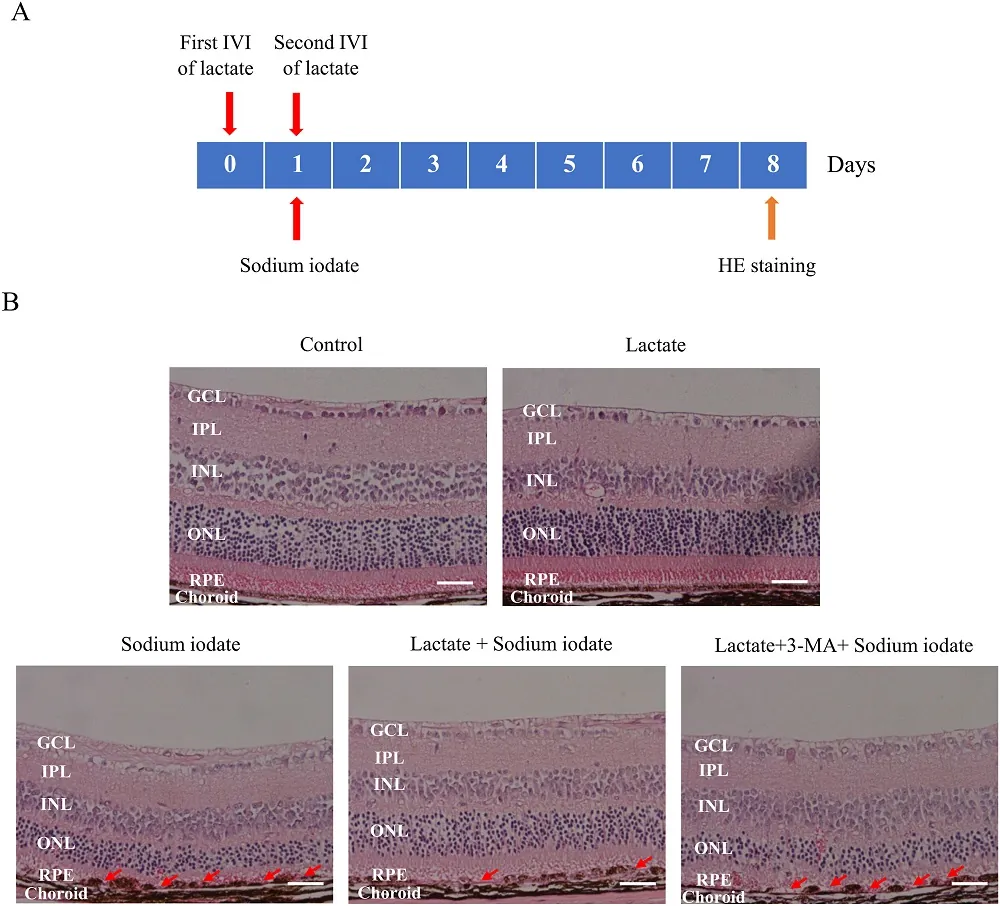
Exploring Autophagy to Fight AMD: Publishing in Free Radical Biology and Medicine, a team of Chinese researchers has investigated the potential role of autophagy in fighting oxidative stress and potentially staving off age-related macular degeneration (AMD).
 Short Bouts of Vigorous Activity May Reduce Mortality Risk: In a study published in Nature Medicine, scientists have shown that short bouts of everyday vigorous physical activity, such as stair climbing, are associated with a considerable reduction in mortality risk, especially in cardiovascular mortality.
Short Bouts of Vigorous Activity May Reduce Mortality Risk: In a study published in Nature Medicine, scientists have shown that short bouts of everyday vigorous physical activity, such as stair climbing, are associated with a considerable reduction in mortality risk, especially in cardiovascular mortality.
Fighting Osteoporosis Through Cellular Signaling: A paper published in Experimental Gerontology has detailed how a bacterially derived compound may be useful in fighting osteoporosis. This research shows that there may be a way to dig deeper into the root causes of osteoporosis.
 Transcriptome-Wide Organization Changes in Aging: In a new study published in Nature Aging, researchers have shown that aging is associated with a decreased expression of long transcripts over multiple tissues across several animal species. This study uncovered a conserved molecular feature of age-associated global transcriptome changes.
Transcriptome-Wide Organization Changes in Aging: In a new study published in Nature Aging, researchers have shown that aging is associated with a decreased expression of long transcripts over multiple tissues across several animal species. This study uncovered a conserved molecular feature of age-associated global transcriptome changes.
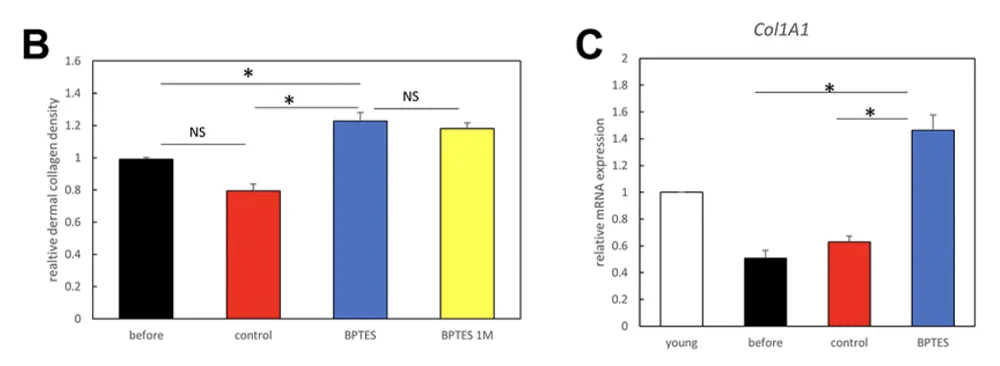
Clearing Out Senescent Cells Rejuvenates Human Skin: Using a novel senolytic drug, scientists have successfully eliminated senescent cells in human skin transplanted into mice. The treatment led to prolonged skin rejuvenation. It works by inhibiting the enzyme glutaminase, which is essential for the survival of senescent cells.
 A Connection Between Laminal Dysfunction and Heart Weakness: A paper published in Nature Aging has explained how changes to the lamina contribute to heart weakness in model organisms. Lamin proteins enclose the nucleus in the lamina, the cellular envelope that contains and protects DNA.
A Connection Between Laminal Dysfunction and Heart Weakness: A paper published in Nature Aging has explained how changes to the lamina contribute to heart weakness in model organisms. Lamin proteins enclose the nucleus in the lamina, the cellular envelope that contains and protects DNA.
Lifespan extension in female mice by early, transient exposure to adult female olfactory cues: These data provide support for the idea that very young mice are susceptible to influences that can have long-lasting effects on health maintenance in later life, and provide a potential example of lifespan extension by olfactory cues in mice.
Inducible Pluripotent Stem Cell-Derived Small Extracellular Vesicles Rejuvenate Senescent Blood–Brain Barrier to Protect against Ischemic Stroke in Aged Mice: In aged stroke mice, iPSC-sEVs significantly mitigated BBB integrity damage, reduced the following infiltration of peripheral leukocytes, and decreased the release of pro-inflammatory factors from the leukocytes, which ultimately inhibited neuronal death and improved neurofunctional recovery.
Ex vivo manufacturing of platelets: beyond the first-in-human clinical trial using autologous iPSC-platelets: This review summarizes current findings on the ex vivo generation of iPSC-PLTs that led to the iPLAT1 study and beyond.
Young plasma transfer recovers decreased sperm counts and restores epigenetics in aged testis: The researchers aimed to show whether blood plasma transfer has an effect on DNA methylation and spermatogenetic cell development.
Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration: As senescent cells also accumulate in human muscles, these findings open potential paths for improving muscle repair throughout life.
Targeting anti-apoptotic pathways eliminates senescent melanocytes and leads to nevi regression: These data highlight the important role of redundant anti-apoptotic mechanisms for the survival advantage of senescent melanocytes and the proof-of-concept for a non-invasive combination therapy for nevi removal.
A molecular signature defining exercise adaptation with ageing and in vivo partial reprogramming in skeletal muscle: Considering reduced biological age according to DNA methylome analysis, high-volume exercise training can be classified as an epigenetic reprogramming stimulus.
DNA methylation GrimAge version 2: GrimAge version 2 is an attractive epigenetic biomarker of human mortality and morbidity risk.
Effect of dietary inflammatory potential on the aging acceleration for cardiometabolic disease: A population-based study: In people with CMD, potentially inflammatory diets were associated with age acceleration.
Association between gut microbiota and longevity: a genetic correlation and mendelian randomization study: This study found evidence that gut microbiota is causally associated with longevity, or vice versa, providing novel clues for understanding the roles of gut microbiota in aging development.
Musical and multilingual experience are related to healthy aging: better some than none but even better together: Musical and multilingual experiences are related to healthy aging, especially when combined, which supports the suggestion that a broader spectrum of lifetime experiences relates to cognitive reserve.
Quantifying the benefits of inefficient walking: Monty Python inspired laboratory based experimental study: Adults could achieve 75 minutes of vigorous intensity physical activity per week by walking inefficiently for about 11 min/day.
Gut microbiota of the young ameliorates physical fitness of the aged in mice: These results provide solid evidence that the gut microbiota from the young improves the vitality of the aged.
News Nuggets
BioAge Announces Positive Results Against Muscle Atrophy: BioAge Labs has concluded a Phase 1b study in which human volunteers undergoing 10 days of bed rest were shown to have their related muscle atrophy significantly attenuated by BGE-105.
Turn Biotechnologies Changes Paradigm in Skin Rejuvenation: Turn Biotechnologies has unveiled data at four industry conferences suggesting that a single ERA treatment may be more effective than combination therapies used today. Biomarker analysis demonstrates ERA’s regenerative impact on fibroblast proliferation and collagen VII production.
Coming Up
VitaDAO Longevity Hackathon: On January 20-22, the VitaDAO hackathon, powered by LongHack, will commence. This is a forum for innovators to present ideas and solutions for behavioral health and wellness. Monetary prizes will be awarded. Lifespan.io is an official partner of this event.