The concern that rejuvenation biotechnologies might cause social disparity and further widen the gap between rich and poor is one of the most commonly raised ones, probably second only to concerns of overpopulation. Like many others, this concern may appear valid at first, but it does not survive careful analysis.
Anti-aging sticker shock
The underlying assumption of this argument is that rejuvenation therapies would be so very expensive that only rich people would be able to afford them, thus fracturing the world into ever-young, ever-healthy rich people and the poor, sick, old people with no access to these technologies. It is very likely that rejuvenation therapies will be quite expensive (at least initially) due to a number of factors.
However, before we delve deeper into the details of this discussion, let’s remind ourselves of an important but possibly understated fact: rejuvenation biotechnologies are life-saving medical treatments that are meant to prevent age-related diseases and allow people to maintain good health throughout their lives. In this sense, they are no different from antiviral or cancer therapies, which are currently prescribed and administered by doctors in appropriate healthcare facilities. So, just the same as any other life-saving treatments, the price is irrelevant in the face of the benefits that they would confer to us, and it is certainly not a valid reason not to develop them.
However, even if we can initially assume a high cost for rejuvenation biotechnologies, we need to keep in mind that new technologies generally start off as very expensive and eventually become affordable and widespread. For instance, it took only 15 years for the full genome sequencing cost to drop from $100 million to $300, making personalised medicine reality globally.
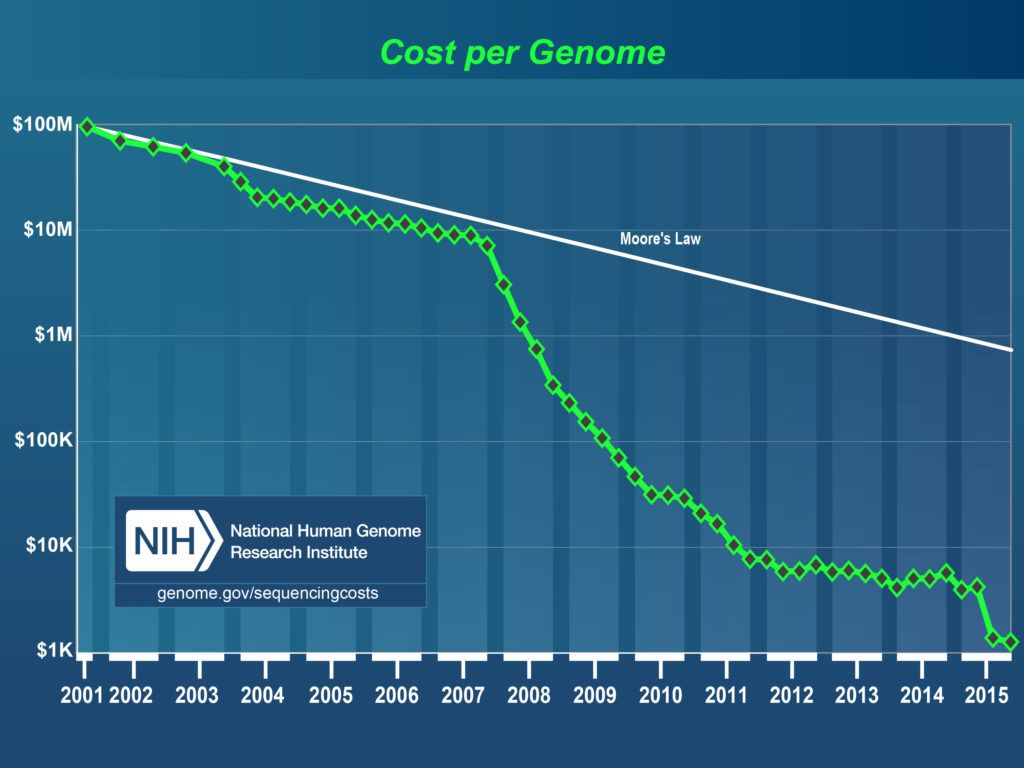
Fig 1. The Cost of Sequencing a Human Genome change, 2001-2015. Source: National Human Genome Research Institute
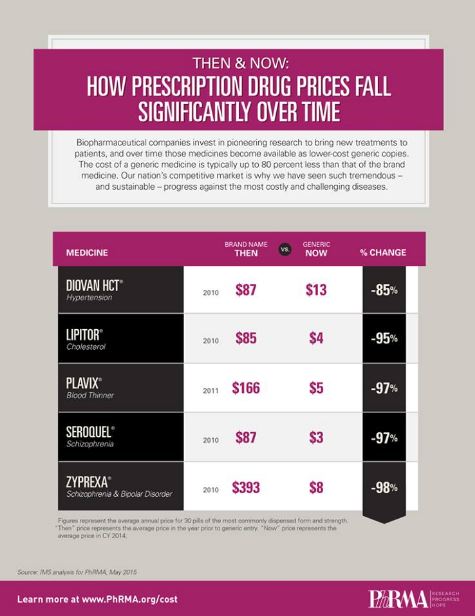
In the field of medicine, there are several other examples of this same trend of falling cost and prices. The drug metformin, which is used for the treatment of type 2 diabetes (and probably the first drug to slow down aging in healthy people, which is currently the subject of the TAME clinical trial), was initially expensive but eventually its price plummeted to a few dollars. Its price fell from $1.24 per tablet in 2002 to 31 cents in 2013. The infographic below shows some other examples of drugs that were once expensive and have subsequently fallen in cost.
Similarly, improvements in technology have drastically reduced the costs of research diagnostics, and the advent of remote technology has allowed a cost reduction for both patients and hospitals, as specialists can be contacted at a distance. For example, hospitals do not need to have radiologists on location all the time. Instead, they can instead remotely send them patient data for analysis and thus only pay for each individual service; this, in turn, implies potentially cheaper services for the patients as well.
Even gene therapies, stem cell therapies and immunotherapies may soon become cheaper, less time-consuming, and consequently more widely available thanks to innovations such as this new semi-automated benchtop system, which produces modified cells in sufficient numbers very rapidly at lower cost, or automated systems for cell therapies and immunotherapies with similar production streamlining and potential cost reduction [1-5].
Outside of the field of medicine, a classic example of dropping prices is that of computers and electronics. At the beginning of the computer age, computers were huge pieces of machinery with very limited capabilities, and only few institutions in the world could afford having a few of them at best. However, as technology improved, the production of computers (and that of electronics in general) has become easier, cheaper, and more efficient, to the point that today we all walk around with tiny computers—smartphones—in our pockets, and these computers are hundreds of times more powerful than those used by NASA to send the first people on the Moon.
Similarly, cars used to be too expensive for an average person to afford. Today, not only are cars ubiquitous and largely affordable, but even the newer, cutting-edge electric cars (for example, Tesla cars) are reaching the same price range as regular cars, despite being relatively new on the market. Another example of the high-tech sector is that of 3D printers. A mere decade ago, a 3D printer cost around $100,000; today, one can buy a good 3D printer for less than $500—a price drop of 95.5%.
Even more interestingly, this trend extends further to other areas of technology, such as the food industry. In 2013, the first lab-grown burger cost $325,000; a mere two years later, the cost had dropped to around $11. Even though the company estimates that it would take two decades to turn the burger into a viable product, other companies have recently shown confidence that this can be achieved on a much shorter time scale.
For example, SuperMeat hopes to have its first lab-grown meats available in stores in only five years, for the same price as regular meat or less. All of these examples show that technology typically becomes much cheaper as time goes by; there is no reason to believe the same would not be true of rejuvenation technologies, especially when one takes into account an extremely strong economic motivator.
Rejuvenation would be the largest industry in historyThe market for rejuvenation biotechnologies would be the largest in history, and, indeed, some investors, such as billionaire investment expert Jim Mellon, are already taking notice. Every single person in the world is aging and is thus a potential customer. It is of course very likely that people who have wealth, and therefore greater means, will obtain cutting-edge technology first (as we have seen repeatedly throughout history) before everyone else. This has always been the case, and it has always been that, shortly thereafter, such technology and access has become widespread.
However, one should consider that those early adopters are playing “guinea pig” and, in effect, are paving the way for the masses and helping developers offset the costs incurred during development, as they are paying premium prices for early access to these technologies.
Socio-economic considerations
If, for the sake of argument, we assume that rejuvenation biotechnologies could somehow be an exception to the trend of falling prices in technology, we would need to decide whether people ending up paying for their own rejuvenation therapies is more of a realistic scenario than governments subsidizing the treatments, partly or wholly.
The majority of countries in the world have universal healthcare systems that take care of their citizens’ or residents’ health needs either for free or for a nominal fee. These costs are offset by taxes, which ensure the health service is able to provide this level of care to all.
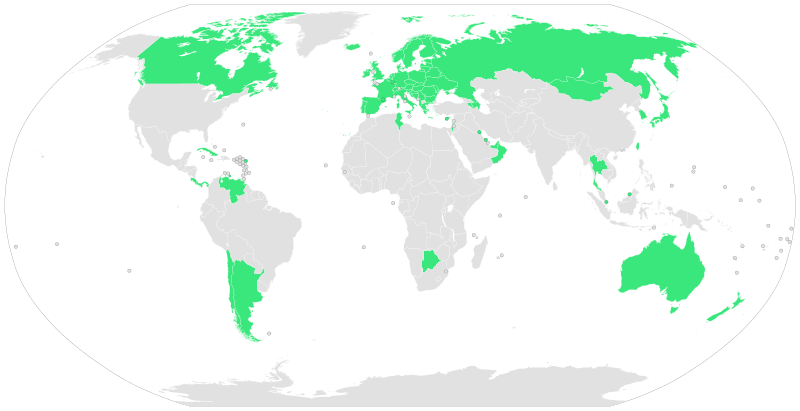
Fig 2. 2009 Countries with universal Healthcare Source: wikimedia
Other countries, particularly the United States, do not have a universal healthcare system; citizens or residents of such countries generally have private health insurance to cover their medical expenses, and the coverage of each insurance plan may vary substantially, depending on what each individual subscriber can afford.
In many ways, aging can be considered to be a chronic, progressive, and fatal disease (indeed, some researchers do [6]) of the type that insurance companies would likely not be willing to pay medical expenses for. However, we should take into account that its progression is remarkably slow. It generally takes decades for age-related diseases to manifest, so it would probably make little sense for people under forty years of age to include rejuvenation treatments in their health insurance. As a matter of fact, the very nature of the problem may call for an entirely different insurance strategy.
Presently, insurance policies are drawn up to cover the cost of potential risks that might lie ahead; this is the very reason why more likely risks make for more expensive policies and vice-versa. One is not guaranteed to get all age-related diseases—simply because one or few of them manage to kill us before we can get the others—but comprehensive, preventative rejuvenation necessarily needs to address all aspects of biological aging that may lead to any such ailments; in this sense, aging is a disease that we all suffer from, and we will all suffer from each of the age-related diseases if we live long enough.
Therefore, aging is not a potential risk but rather a certain fate that will strike each of us differently, depending on individual circumstances. While the market share of each insurance company offering rejuvenation policies would be huge, such policies could not logically treat aging in the same way they treat potential risks. An insurance company will pay for a customer’s stolen car—or medical expenses for a certain disease—if and when the car has been stolen—or when the disease has struck.
However, aging is a lifelong process that needs to be addressed in a preventative fashion [7] [8]; at no specific point could we say “aging has struck the patient now, but not before.” This suggests that private insurance may not the be right way to go; instead, we should consider this matter from the point of view of the government.
Health costs in the current system are unsustainableHealth expenditures for the elderly currently constitute a considerable burden on a country’s economy. Although the elderly have already contributed wealth to society when they were younger, they often stop doing so when they retire. Furthermore, they receive a pension from the government and will do so for the rest of their lives. While, in theory, pensions should already have been paid for by the elderly themselves with their work earlier in their lives, things are quite different in practice, and some countries are already increasing the retirement threshold further.
This is an unsustainable strategy, because barring radical interventions—such as rejuvenation therapies—there is a point past which elderly people simply are not healthy enough to work. As a matter of fact, the cost of medical expenses for the elderly grows dramatically each year, and it is also to be noted that geriatric treatments lead to no significant results, both in terms of overall individual well-being and fitness for work, and they become increasingly less effective as the patients grow older. As a result, most individuals produce no wealth and their deteriorating health causes increasingly significant expenses during the last year or two of life and further strain on the economy [9].
The desired result of rejuvenation therapies leads to a much better scenario. If rejuvenation therapies are reapplied with proper timing, no individual would ever reach a state of age-related decay and poor health that could make him or her unfit for work. Consequently, the costs of treating age-related diseases using current medicine could be reduced with the arrival of more robust therapies offered by rejuvenation biotechnology. Such rejuvenation therapies aim to prevent a plethora of diseases before they manifest, potentially saving money. However, even if the costs are the same and we are simply trading one set of medicines for another, the benefit to health, quality of life and productivity makes it more than worth it regardless.
Retirement and increased lifespans
When considering retirement in relation to rejuvenation biotechnology, we should consider the two possible scenarios that may arise. The first scenario is the simple increase of lifespan beyond the current limits – this could be a few years or even a decade or two of healthy life. The second scenario is that of negligible senescence, in which medicine allows indefinite lifespans via periodic repair of the various damages and dysfunctions aging causes. Let’s take a look at both of these possibilities and consider the benefits and changes society may encounter as a result.
Scenario 1: Increased lifespans. In a situation where additional healthy years were added to the human lifespan, an immediate benefit to society would be that people could retire later in life and continue to contribute economically and remain productive for longer. This would reduce the costs of pensions and help bolster the economy. Ultimately, in this scenario people would still retire as they already do, but later in life and with more healthy years to enjoy.
Scenario 2: Negligible senescence. In the more extreme case of negligible senescence, people would, in principle, be able to work indefinitely, regardless of their age, thus producing wealth nearly constantly. For this reason, retirement would likely change from a permanent cessation of working to the need for a break longer than a normal holiday, perhaps similar to a long-term work sabbatical. Pensions might even become optional, with workers taking sabbatical breaks from work that could last for years, allowing people to retrain and reinvent themselves.
Another remarkable benefit would be that the government would see drastically reduced costs, which are traditionally spent on pensions, as people would no longer retire in the traditional sense. It might be the case that the government would perhaps replace traditional pension schemes with sabbatical or UBI schemes to allow workers to accrue money over time to take such a break from work. If the State did not do this, then private enterprise might provide such a service.
In short, this second scenario has the potential to turn the current situation on its head—we would go from having high morbidity of age-related diseases that disable the elderly preventing them from being productive and causing major public expenses, to having productive elderly who contribute to the economy and whose health needs are little-to-no burden on it.
It’s easy to see how it would be far more convenient for any given State to pay for the periodical rejuvenation treatments of its citizens rather than maintaining the current state of affairs.
Apart from economical considerations, we must also keep in mind that, as said, rejuvenation therapies are life-saving medical treatments that prevent age-related diseases from ever manifesting. As such, ensuring their development and widespread access is among the objectives of WHO, according to its Constitution. The WHO Constitution says that each government is responsible for the health of its residents, and the attainment of the highest health standards is the very objective of WHO itself.
The WHO definition of ‘health’ is a ‘state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’. Rejuvenation therapies match this definition perfectly: they would eliminate age-related diseases entirely and enable people to enjoy a normal life regardless of their age, without the limitations currently imposed on the elderly by their constantly declining health. They would improve the physical, mental, and social well-being of all people.
The WHO Constitution further states that ‘[t]he enjoyment of the highest attainable standard of health is one of the fundamental rights of every human being without distinction of race, political belief, economic or social condition. In other words, because of the health standard improvements they would cause, rejuvenation therapies would be a basic right of everyone, and both local governments and WHO would have a responsibility to do all in their power to make sure this right is respected. (As a side note, this last paragraph of the Constitution would be better if it said that no distinction of age should be made either).
If not everyone can have it, then no one should have it?
Thus far, we’ve argued that while rejuvenation therapies may well be expensive at the beginning, they would be government subsidized and would likely become cheaper and cheaper as the underlying technology improves. However, what if this prediction were wrong?
If everyone was supposed to pay for their own, expensive rejuvenation, then being indefinitely young would indeed be only for the rich, and the rich-poor gap would become even worse than it already is. It’s easy for people in developed countries to feel guilty about the privileges they have with respect to people in developing countries. Everlasting youth would be quite something to feel guilty about if not everyone had it. Not developing rejuvenation might thus prevent first-world people from having yet another thing to feel guilty about, but that’s all it would do; it would not help third-world people in any way.
Let’s assume that we found out that rejuvenation would be so expensive that only very rich people could afford it, and, for this reason, we decided not to develop it at all. How would this affect the poor? We didn’t develop rejuvenation, so nobody got it, including the poor. If we had developed rejuvenation, the poor wouldn’t have gotten it anyway, because it would have been to expensive for them to afford. So either way, nothing changed for the poor, and they certainly didn’t benefit from rejuvenation not existing.
What about the rich? If we didn’t develop rejuvenation for fear of increasing the gap between rich and poor, then the rich – like the poor – would keep getting sicker as they aged. Surely this didn’t make the gap larger, but it didn’t make it any narrower either. Not developing rejuvenation (or any other new technology, for that matter) doesn’t help the poor, and doesn’t close the rich-poor gap. If we think about it, we see that simply closing the gap isn’t enough. What really matters is how we close it. If developed countries gave up on all their comforts and wealth and became like the developing countries, then the gap would be closed, but no one would benefit from it. The only sensible way to close the rich-poor gap is making the poor better off, not the rich worse off.
ConclusionSo, no, not developing rejuvenation isn’t the way to go. Rejuvenation, like anything that can improve human life, needs to be developed. The concern that rejuvenation might become a privilege of the rich only is legitimate, but the way to avoid this scenario is not to give up on rejuvenation entirely but rather to work hard to ensure that it becomes as widespread and affordable as basic medicine.
Each and every one of us has the power to do something in this sense – be it by working on the necessary science, supporting it financially, lobbying to change relevant regulations, or even just spreading the word and let everyone know about this possibility. Our journey towards a future free from age-related diseases for everyone will be as short – or as long – as we will make it.
Literature
[1] Konagaya, S., Ando, T., Yamauchi, T., Suemori, H., & Iwata, H. (2015). Long-term maintenance of human induced pluripotent stem cells by automated cell culture system. Scientific reports, 5.
[2] Rafiq, Q. A., Twomey, K., Kulik, M., Leschke, C., O’Dea, J., Callens, S., … & Murphy, M. (2016). Developing an automated robotic factory for novel stem cell therapy production. Regenerative medicine, 11(4), 351-354.
[3] Chen, V. C., Ye, J., Shukla, P., Hua, G., Chen, D., Lin, Z., … & Hsu, D. (2015). Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem cell research, 15(2), 365-375.
[4] Conway, M. K., Gerger, M. J., Balay, E. E., O’Connell, R., Hanson, S., Daily, N. J., & Wakatsuki, T. (2015). Scalable 96-well plate based iPSC culture and production using a robotic liquid handling system. Journal of visualized experiments: JoVE, (99).
[5] Granzin, M., Soltenborn, S., Müller, S., Kollet, J., Berg, M., Cerwenka, A., … & Huppert, V. (2015). Fully automated expansion and activation of clinical-grade natural killer cells for adoptive immunotherapy. Cytotherapy, 17(5), 621-632.
[6] Bulterijs, S., Hull, R. S., Björk, V. C., & Roy, A. G. (2015). It is time to classify biological aging as a disease. Frontiers in genetics, 6.
[7] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[8] Kennedy, B. K., Berger, S. L., Brunet, A., Campisi, J., Cuervo, A. M., Epel, E. S., … & Rando, T. A. (2014). Aging: a common driver of chronic diseases and a target for novel interventions. Cell, 159(4), 709.
[9] Scitovsky, A. A. (2005). “The High Cost of Dying”: What Do the Data Show?. Milbank Quarterly, 83(4), 825-841.











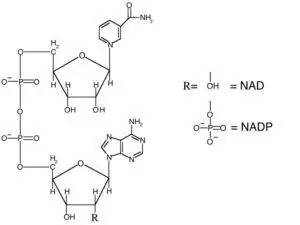 Nicotinamide adenine dinucleotide (NAD) is a dinucleotide, meaning that it consists of two nucleotides joined by their phosphate groups. One nucleotide contains an adenine base, and the other contains nicotinamide. NAD is found in an oxidized form and a reduced form, abbreviated as NAD+ and NADH respectively.
Nicotinamide adenine dinucleotide (NAD) is a dinucleotide, meaning that it consists of two nucleotides joined by their phosphate groups. One nucleotide contains an adenine base, and the other contains nicotinamide. NAD is found in an oxidized form and a reduced form, abbreviated as NAD+ and NADH respectively.




 Moody’s company has received both public and private funds for over 3.2 million dollars, and his efforts
Moody’s company has received both public and private funds for over 3.2 million dollars, and his efforts 
 Surprisingly, when Jeanne was 118 years old, cognitive tests revealed that she scored within the normal range and lacked signs of dementia. However, by that time she was physically frail and required a wheelchair.
Surprisingly, when Jeanne was 118 years old, cognitive tests revealed that she scored within the normal range and lacked signs of dementia. However, by that time she was physically frail and required a wheelchair.
 When we celebrate Jeanne Calment’s birthday, let’s remember that the most human thing to do is not just to remember the record but to try hard to surpass it. Jeanne died in 1997, so she could not benefit from the advances in aging research that are available to us now.
When we celebrate Jeanne Calment’s birthday, let’s remember that the most human thing to do is not just to remember the record but to try hard to surpass it. Jeanne died in 1997, so she could not benefit from the advances in aging research that are available to us now.

