In the Journal of Inflammation, researchers from Johns Hopkins University have published a detailed review of the relationship between brain inflammation and the principal diseases of dementia.
A focus on genetics and environment
One out of twenty Americans over 85 have Parkinson’s disease [1], and seven out of twenty have Alzheimer’s [2]. While genes are obviously a factor in this prevalence, and environmental factors are another large factor, this review focuses on a combination of the two: gene-by-environment (GxE) interactions. This is the idea that the two are heavily intertwined; in other words, the way in which a biological process responds to its environment is dictated by its genes [3].
The researchers make it clear that it is impossible to reduce most instances of these neurodegenerative diseases down to specific environmental or genetic triggers; they can be associated with an increased likelihood of such diseases, such as the APOE4 allele, but there are only a handful of specific mutations that are known to consistently and directly result in neurodegeneration.
This review focuses on the GxE relationships involved in inflammation. This interaction between genes and the environment is very well known: immune cells, whose activation is often directly related to environmental insults such as bacteria, recognize pathogens and signs of damage and so begin the process of inflammation and later healing.
However, as the researchers note, the markers that are most commonly associated with tissue inflammation, such as redness and swelling, do not appear in the central nervous system, whether that inflammation is chronic or acute. Instead, the activation of the macrophages known as microglia send chemical messages to astrocytes, and the combination of these cells can send signals, some of which are neurotoxic and some of which are neuroprotective.
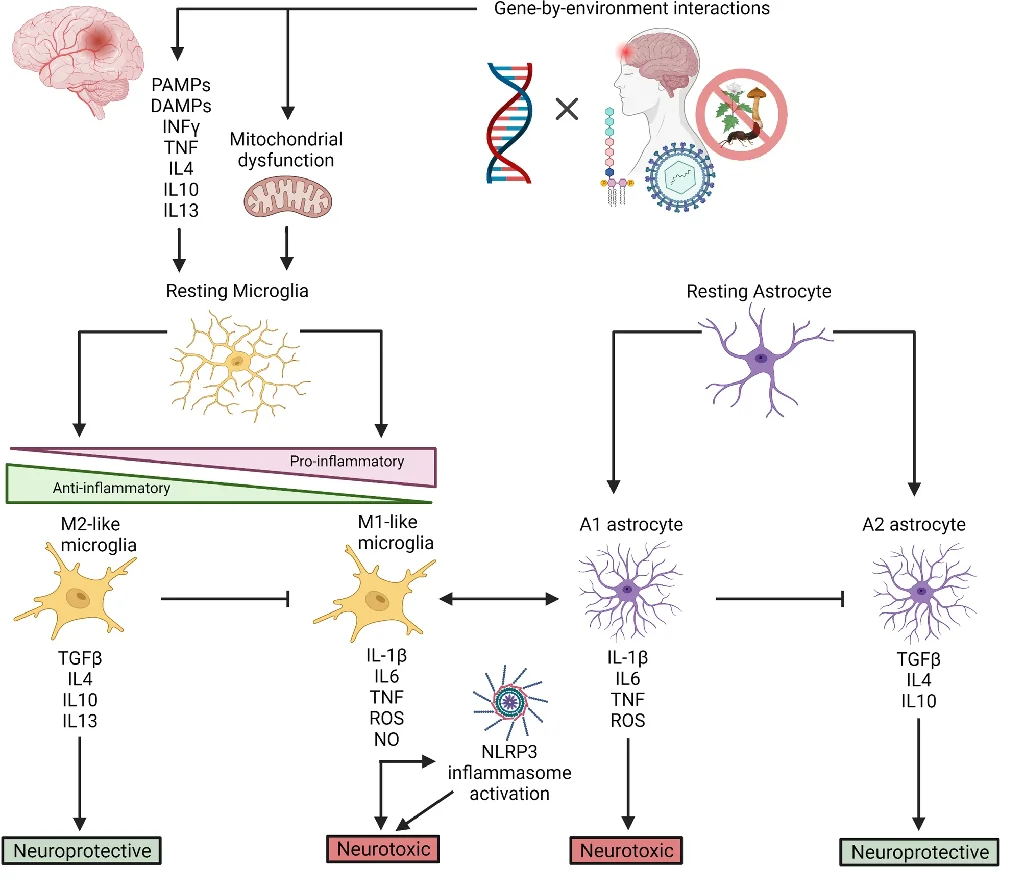
These environmental effects can also damage the genome, directly through mutation and indirectly through cellular stress, causing a wide variety of biological effects that become clear in -omics analysis [4]. Chronic inflammation is a well-known cause of other chronic ailments, to the point that this relationship can be found in well-studied encyclopedias [5]. As it is clear that the increase in neuroinflammation is associated with these diseases [6], the reviewers sought to determine precisely how this happens in two of the most well-known neurodegenerative disorders: Alzheimer’s and Parkinson’s.
Inflammation, genes, and Alzheimer’s
The researchers note that there are a few mutations to the amyloid precursor protein and presenilin genes that are very strongly associated with Alzheimer’s. However, people with these mutations are likely to get the disease before the age of 60, and these cases are fortunately very rare [7].
However, the APOE4 allele is rather common, and people with one copy of this variant have triple the Alzheimer’s risk of people without it; people with two copies have eight times the risk. The APOE2 allele confers some protection [8].
Other than these, there are more than 50 known genetic risk factors for Alzheimer’s disease, and 30 of them have been noted in more than one study [9]. While the exact relationship between all of these genes and the disease is hard to determine, neuroinflammation is a frequent contributor.
Inflammation, mediated by microglia, leads to tissue death and damage in Alzheimer’s, and while microglia can clear amyloid beta, doing so often causes more pro-inflammatory factors to be released, making the problem worse [10]. Reactive oxygen species (ROS) are also known to contribute to Alzheimer’s, and inflammation can lead to ROS in the brain, which can lead to more inflammation [11].
As expected, APOE4 is more associated with inflammation than other APOE allelles [12]; more neurotoxic factors were found in the plasma of people carrying it. An exact biochemical cause has been theorized: this particular form of inflammation has been reported to be driven by cholesterol, and the APOE4 mutation does not bind to cholesterol as well [13], thus leading to its accumulation in microglia. Mutations to TREM2, which is central to microglial function [14], and many other immunomodulatory genes have also been found to play roles in Alzheimer’s.
Inflammation, genes, and Parkinson’s
While Parkinson’s can run in families, only 15% of Parkinson’s patients have this sort of genetic transmission listed as their primary cause [15]. Often, the genetic variants are of the α-synuclein protein itself; if this protein is misfolded or overexpressed, the link to Parkinson’s is direct, without any immune system involvement.
However, with Parkinson’s later in life, the situation is similar to Alzheimer’s. A full 90 potential genetic factors have been found [16], and the researchers note that the specific neurons affected by Parkinson’s are uniquely vulnerable to inflammation; at least in mice, the area contains more microglia than anywhere else in the brain [17]. Under normal circumstances, the chemical neuromelanin is protective; however, as deteriorating neurons in the area release it into the extracellular environment, microglia treat it as a foreign chemical and release inflammatory markers when consuming it [18]; the iron in the neuromelanin also causes ROS through hydrogen peroxide [19].
The SNCA gene has been singled out as a potential cause. Mutations to this gene cause microglia to recognize the protein’s aggregates as foreign, activating them and causing further inflammation [20], just as amyloid beta causes microglial overactivation in Alzheimer’s. The LRRK2 gene was also identified, as it is associated with mitochondrial function and cytokine response.
Conclusion
Even though this review goes into exhaustive detail, finding every single combination of genetic predisposition and environmental insult is impossible. Rather, for any potential therapy to be developed and transmitted based on this research, it must modulate the effect of microglia and reduce inflammation while retaining these immune cells’ ability to protect the brain. Given the frequency and intensity of immune responses in these crippling and deadly diseases, it is not unreasonable to believe that a successful future combination therapy for Alzheimer’s or Parkinson’s will have an anti-inflammatory aspect.
Literature
[1] Reeve, A., Simcox, E., & Turnbull, D. (2014). Ageing and Parkinson’s disease: why is advancing age the biggest risk factor?. Ageing research reviews, 14, 19-30.
[2] Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & dementia, 15(3), 321-387.
[3] Hunter, D. J. (2005). Gene–environment interactions in human diseases. Nature reviews genetics, 6(4), 287-298.
[4] Hasin, Y., Seldin, M., & Lusis, A. (2017). Multi-omics approaches to disease. Genome biology, 18(1), 1-15.
[5] Fleit, H. B. (2014). Chronic inflammation.
[6] Sochocka, M., Diniz, B. S., & Leszek, J. (2017). Inflammatory response in the CNS: friend or foe?. Molecular neurobiology, 54(10), 8071-8089.
[7] Van Cauwenberghe, C., Van Broeckhoven, C., & Sleegers, K. (2016). The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genetics in Medicine, 18(5), 421-430.
[8] Avramopoulos, D. (2009). Genetics of Alzheimer’s disease: recent advances. Genome medicine, 1(3), 1-7.
[9] Wightman, D. P., Jansen, I. E., Savage, J. E., Shadrin, A. A., Bahrami, S., Holland, D., … & Posthuma, D. (2021). A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nature genetics, 53(9), 1276-1282.
[10] Hickman, S. E., Allison, E. K., & El Khoury, J. (2008). Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. Journal of Neuroscience, 28(33), 8354-8360.
[11] Popa-Wagner, A., Mitran, S., Sivanesan, S., Chang, E., & Buga, A. M. (2013). ROS and brain diseases: the good, the bad, and the ugly. Oxidative medicine and cellular longevity, 2013.
[12] Fan, Y. Y., Cai, Q. L., Gao, Z. Y., Lin, X., Huang, Q., Tang, W., & Liu, J. H. (2017). APOE ε4 allele elevates the expressions of inflammatory factors and promotes Alzheimer’s disease progression: a comparative study based on Han and She populations in the Wenzhou area. Brain research bulletin, 132, 39-43.
[13] Xu, Q., Brecht, W. J., Weisgraber, K. H., Mahley, R. W., & Huang, Y. (2004). Apolipoprotein E4 domain interaction occurs in living neuronal cells as determined by fluorescence resonance energy transfer. Journal of Biological Chemistry, 279(24), 25511-25516.
[14] Neumann, H., & Daly, M. J. (2013). Variant TREM2 as risk factor for Alzheimer’s disease. N Engl J Med, 368(2), 182-4.
[15] Tran, J., Anastacio, H., & Bardy, C. (2020). Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Parkinson’s disease, 6(1), 1-18.
[16] Nalls, M. A., Blauwendraat, C., Vallerga, C. L., Heilbron, K., Bandres-Ciga, S., Chang, D., … & Rizig, M. (2019). Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. The Lancet Neurology, 18(12), 1091-1102.
[17] Lawson, L. J., Perry, V. H., Dri, P., & Gordon, S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 39(1), 151-170.
[18] Hirsch, E. C., Vyas, S., & Hunot, S. (2012). Neuroinflammation in Parkinson’s disease. Parkinsonism & related disorders, 18, S210-S212.
[19] Zecca, L., Casella, L., Albertini, A., Bellei, C., Zucca, F. A., Engelen, M., … & Sarna, T. (2008). Neuromelanin can protect against iron‐mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. Journal of neurochemistry, 106(4), 1866-1875.
[20] Hoenen, C., Gustin, A., Birck, C., Kirchmeyer, M., Beaume, N., Felten, P., … & Heurtaux, T. (2016). Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: stronger effects of the A53T mutant. PloS one, 11(9), e0162717.