Human physiology contains numerous functional redundancies, with multiple mechanisms to address and back up critical processes. This is especially evident in the control of cell division, which is essential for growth, development, wound healing, regeneration, and cancer prevention [1, 2]. Telomere attrition is one of several regulatory mechanisms that ensure that cells do not divide uncontrollably.
However, while telomere attrition helps prevent cancer, it also leads to cellular suicide (apoptosis) and senescence, which reduces the body’s ability to function and its regenerative power. This loss of regenerative capacity is a primary aging driver, as senescent cells accumulate and impair the body’s ability to repair and renew tissues, and apoptosis depletes the body of functional cells [3]. Consequently, telomere attrition is one of López-Otín’s four primary hallmarks of aging [4].
Telomere shortening is especially problematic in cells that divide frequently, including numerous immune, skin, intestine, and hair cells. When telomere erosion reaches the Hayflick limit, typically after 50 cell divisions, the cell undergoes apoptosis or becomes senescent [5, 6].
Ripple effects on other hallmarks of aging and disease
As telomeres shorten, their protective abilities diminish, leading to increased chromosome end-to-end fusions, loss of genetic information, and genomic instability [7]. Critically short telomeres trigger a persistent DNA damage response, exacerbating genomic instability by activating repair pathways that may introduce mutations or structural chromosome aberrations [8].
Telomere shortening is also associated with changes in the organized combination of DNA and proteins that spool it (chromatin) along with chromosome ends and other genomic regions, leading to altered gene expression patterns and epigenetic drift. This influences the expression of genes involved in maintaining epigenetic marks, resulting in widespread epigenetic changes [9]. The persistent DNA damage response caused by short telomeres can activate stress response pathways, including those that affect protein homeostasis. This can result in the accumulation of misfolded or damaged proteins. Furthermore, senescent cells induced by telomere attrition secrete pro-inflammatory cytokines, proteases, and other factors, known as the senescence-associated secretory phenotype (SASP), which can disrupt proteostasis and promote tissue dysfunction [10].
The cellular senescence induced by telomere attrition also leads to alterations in metabolic pathways, including nutrient-sensing pathways like mTOR, AMPK, and insulin/IGF-1 signaling, which can affect cellular growth and metabolism, contributing to aging. Senescent cells can also contribute to systemic insulin resistance through the SASP, impacting nutrient sensing and metabolic health [11]. Additionally, short telomeres can lead to mitochondrial dysfunction by increasing oxidative stress, further damaging telomeric DNA and other cellular components. Senescent cells induced by telomere attrition often exhibit impaired mitochondrial function, reducing cellular energy production and increasing reactive oxygen species [12].
Telomere attrition is a primary trigger for cellular senescence. As cells reach their replicative limit due to shortened telomeres [5], they enter a state of permanent growth arrest and adopt the SASP, impacting tissue function and promoting aging. The resulting accumulation of senescent cells contributes to tissue degeneration and dysfunction, promoting the development of age-related diseases [13, 14]. In stem cells, telomere shortening limits their ability to proliferate and maintain tissue homeostasis, leading to stem cell exhaustion [15], which is another hallmark of aging. This reduces tissues’ regenerative capacity, as telomere attrition drives stem cells into senescence, further depleting the stem cell pool and impairing tissue repair and regeneration.

The pro-inflammatory environment created by senescent cells can impair normal cell function and communication, contributing to the overall decline in tissue health and function [14]. Telomere attrition, therefore, drives the development of all eight of the other original hallmarks of aging: genomic instability, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. The progressive shortening of telomeres and the resulting cellular senescence create a cascade of detrimental effects that collectively contribute to aging processes and the onset of age-related diseases.
The number of diseases shown to be directly and indirectly driven by telomere attrition is staggering and includes, but is not limited to, cardiovascular disease [16], cancer [17], neurodegenerative diseases [18], diabetes [19], and arthritis [20]. Additionally, several specific diseases are characterized by telomere dysfunction, such as aplastic anemia [21] and dyskeratosis congenita [22].
Thus, scientists from many disciplines are working to understand how telomere maintenance works and how we can augment the body’s natural processes to better prevent cancer and promote healthy longevity.
Telomeres, enzyme complexes, and signaling pathways
Telomere attrition occurs as a byproduct of cell division and results from “the end replication problem”; the cell machinery that copies DNA has no place to anchor itself while copying the last few building blocks (nucleotides) of a DNA strand, so it doesn’t. This results in a progressive shortening of the chromosome’s telomeres each time the cell divides [23].
At each chromosome’s end is a long stretch of telomeric repeats. A telomeric repeat is a six-nucleotide sequence. Thousands of these repeats are tacked onto the chromosome before the tip is neatly looped and tucked into it to protect the telomere from damage [24].
The attrition process is complex because the body has mechanisms for extending and shortening telomeres. Telomerase is a critical player in telomere length regulation, as it counters the erosion of telomeres by partially rebuilding them after cell division occurs [25]. However, the rate at which this happens can vary greatly depending on individual genetic predisposition, exposure to oxidative damage, and other cellular stressors. A complex array of biomolecules regulates telomere length as the body simultaneously attempts to protect itself from cancer and optimize tissue regeneration capacity [26].
Two major enzyme complexes and several signaling pathways are crucial in the regulation of telomere length, notably the shelterin complex (TRF1, TRF2, TIN2, TPP1, POT1, and RAP1), the telomerase complex (TERT and TERC), its stabilizing proteins (DKC1, NOP10, NHP2, and GAR), and critical signaling pathways, including the DNA damage response pathways (ATM and ATR), the p53 pathway, the c-Myc pathway, the MAPK/ERK pathway, and the TGF-β pathway. When working correctly, these components and pathways ensure genomic stability, cellular longevity, and proper stress and damage response, forming the telomere maintenance and protection backbone [27].
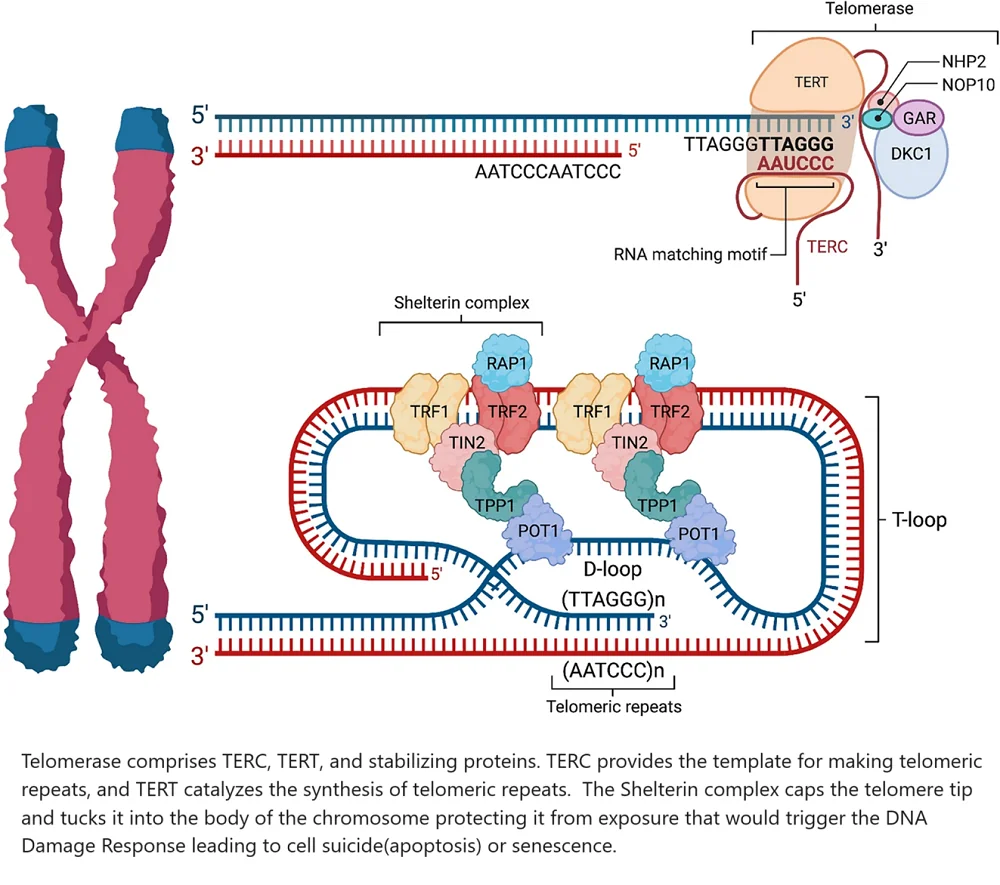
How the telomere maintenance system works
TERC harbors the RNA template needed to make the DNA sequence (TTAGGG) of the telomeric repeat. TERT binds to TERC to catalyze the synthesis of telomeric repeats [28].
The shelterin complex masks telomeres to prevent them from being treated as DNA damage. Without this feature in place, inappropriate DNA repair mechanisms would be triggered [29].
Shelterin’s subcomponents assume several roles. TRF1, TPP1, and POT1 block telomerase from gaining access to the telomere and bind to single-stranded telomeric DNA [30]. TRF2 and RAP1 work together to prevent the activation of the DNA damage response by inhibiting the ATM kinase pathway, which is activated in response to DNA double-strand breaks [31]. TRF2 also tucks the single-stranded telomeric overhang into the body of the chromosome to add additional protection from the body’s DNA damage surveillance mechanisms [32]. TIN2 is the unsung hero that holds the shelterin complex together [33].
Regulation of telomerase in disease and stress
Telomerase regulation is critical for maintaining genomic stability, and its dysregulation is associated with various diseases, including cancer and age-related disorders [34].
TRF1 and TRF2 expression levels can be altered in cancers. Overexpression of TRF2 has frequently been observed in cancer cells, and it facilitates limitless replication by preventing telomere shortening [35]. Under stress conditions, such as oxidative stress, TRF1 and TRF2 can be upregulated to help protect telomeres from damage.
POT1 binds to single-stranded telomeric DNA and regulates telomerase access to the telomere. Mutations in POT1 can lead to dysregulated telomere elongation, contributing to tumorigenesis. During stress, altered binding of POT1 can lead to telomere instability and increased susceptibility to DNA damage [36].
TIN2 is a scaffolding protein stabilizing the shelterin complex. Mutations in TIN2 are linked to telomere syndromes, such as dyskeratosis congenita, which can lead to bone marrow failure and increased cancer risk [37]. TIN2 mutations can impair telomere maintenance under stress, exacerbating telomere shortening and dysfunction [38].
Relevant signaling pathways
ATM and ATR are proteins involved in the cellular response to DNA damage. They respond to double and single-strand DNA breaks, respectively. They are crucial in maintaining genomic stability and orchestrating the DNA damage response (DDR) pathways. TRF2 inhibits ATM signaling at telomeres, preventing inappropriate DNA damage responses [39]. Dysregulation of ATM can lead to genomic instability and cancer [40].
ATR helps maintain telomere integrity under the stress of cell division. POT1 and TRF1 regulate ATR signaling at telomeres [41].
ATM and ATR alike can activate p53, which is a tumor suppressor that can induce cell cycle arrest, apoptosis, or senescence in response to DNA damage [42]. P53 is mutated or inactivated in many cancers, leading to uncontrolled cell proliferation. Telomere dysfunction can activate p53, linking telomere maintenance to tumor suppression. Under telomere stress, p53 is activated to initiate repair or trigger apoptosis, preventing the propagation of damaged cells [43].
c-Myc is an oncogene that can upregulate telomerase reverse transcriptase (TERT) expression, enhancing telomerase activity. Overexpression of c-Myc is common in cancers, leading to increased telomerase activity and enabling immortalization of cancer cells. Cellular stress can modulate c-Myc levels, influencing telomerase activity and telomere maintenance [44].
Conversely, TGF-beta [45] and P38/MAPK can decrease telomerase expression. P38/MAPK is a crucial regulator of the cellular stress response, which explains why people under stress often have abnormally shortened telomeres [46].
This is not a complete picture of cell signaling as it relates to telomere maintenance. However, it does touch on the primary cell signaling pathways that can influence telomere length.
Recent advances
An avalanche of research culminated in 2024 with publications highlighting the critical functions of telomeres and telomerase in immune cells and the central nervous system, emphasizing their impact on cellular aging and neurodegenerative diseases. Several reviews addressed the limitations of using telomeres as biomarkers for chronological age; others explored the genetic factors influencing telomere length and longevity.
Innovative techniques for precise telomere measurement were established; telomere dynamics on healthspan and space exploration were studied. Several studies were also published to address the associations between telomere length, disease prevalence, and environmental factors such as smoking.
Finally, new insights into the age-dependent relationship between telomere length and aging and the potential of telomerase gene therapy for cardiovascular diseases were investigated. These findings underscore telomeres’ complex yet vital role in aging and disease, opening new avenues for therapeutic interventions. What follows are just a handful of research highlights from late 2023 and 2024.
In August 2023, Savage determined that the baseline length of telomeres is determined by both rare and common genetic variants inherited from parents. The study highlighted that rare genetic variants in genes responsible for maintaining telomeres can cause some individuals to have exceptionally short telomeres for their age (less than the 1st percentile).
These conditions, known as telomere biology disorders, are associated with higher risks of bone marrow failure, myelodysplastic syndrome, acute myeloid leukemia, and squamous cell carcinoma in the head, neck, and anogenital regions. Conversely, rare genetic variants that result in unusually long telomeres are linked to increased risks of other cancers, such as chronic lymphocytic leukemia and sarcoma [47]. This research raises significant questions about the virtues of potential telomere-lengthening therapies.
The findings emphasize that having neither short nor long telomeres is crucial for minimizing cancer risk. The study concludes that maintaining a “just right” length of telomeres is essential for protecting against cancer [47].
In October 2023, Harley and colleagues investigated the role of telomerase reverse transcriptase (TERT) and telomere shortening in the central nervous system (CNS). Using CRISPR/Cas9 to create human induced pluripotent stem cells (hiPSCs) with reduced telomerase function, they studied motor neurons and astrocytes. Their findings showed that telomere shortening induced aging-associated characteristics such as increased cellular senescence, inflammation, and DNA damage. This study highlighted TERT’s essential role in neural progenitor cell proliferation and neuronal differentiation, providing a valuable model for studying age-related neurodegenerative diseases [48].
In November 2023, Chebly and colleagues explored aging, focusing on how human immune cells, particularly T-cells, undergo significant changes related to telomeres and telomerase. Their review highlighted the crucial roles of telomeres and telomerase in T-cell differentiation and aging. They noted a strong link between short telomeres, telomerase activation, and various T-cell malignancies. Their comprehensive review of existing literature emphasized the impact of telomere dynamics on health and age-related diseases [49].
In December 2023, Pepke reviewed the role of telomeres as biomarkers for aging and their limitations in predicting chronological age. Despite a correlation between telomere length and age, significant individual variation influenced by genetic and environmental factors limits their predictive reliability. The review emphasized that telomeres should not be used for age estimation, cautioning against misleading efforts in wildlife conservation [50].
In January 2024, Romero-Haro and colleagues investigated the age dependence of the relationship between telomere length and aging in the Japanese quail. They found a robust negative association between telomere length and age in young adults but no such association in older adults. This study suggested that the costs and benefits of telomere maintenance vary throughout life, contributing to the mixed evidence on telomere dynamics and aging [51].
In February 2024, Abdel-Gabbar explored the role of telomeres and telomerase in age-related cardiovascular diseases. Short telomeres activate the DNA damage response (DDR), leading to cell cycle arrest in heart cells. This limited regenerative capacity is linked to conditions like heart failure. The study highlighted the potential of telomerase gene therapy to restore heart cell proliferation and treat cardiovascular diseases [52].
In March of 2024, Torigoe and colleagues examined the FOXO3 gene variant rs2802292 and its relationship with telomere length, telomerase activity, and inflammation. They found that individuals with the longevity-associated G-allele of FOXO3 had longer telomeres and higher telomerase activity. Older female carriers showed a decline in pro-inflammatory IL-6 levels, while older males had increased anti-inflammatory IL-10 levels. This study suggested that FOXO3 promotes longevity through different mechanisms in men and women [53].
In April 2024, Karimian and his team developed telomere profiling, a method of precisely measuring telomere length. They discovered that telomere lengths vary significantly among different chromosome ends and that these lengths are established at birth and maintained as individuals age. Examining telomere lengths in 147 individuals found consistent patterns in specific chromosome ends. Telomere profiling could enhance research and clinical approaches by providing detailed insights into telomere biology and its role in aging and disease [54].
Also in April 2024, Mason and his team discussed the importance of improving human health in light of an aging population. They focused on telomeres, which are protective caps at the ends of chromosomes that shorten with each cell division due to the “end-replication problem.” This shortening is linked to aging and age-related diseases, including reduced fertility, dementia, cardiovascular disease, and cancer. They also discussed the lack of data on how long-duration space missions might affect telomeres and overall health. Insights from the NASA Twins Study have advanced understanding of these effects and could inform strategies for future space missions and human longevity in extreme environments [55].
That is also the month when Liang and his team used a DNA methylation-based telomere length (DNAmTL) estimator to study cancer prevalence and mortality in people with and without HIV. They found that individuals with HIV had shorter DNAmTL, associated with higher cancer prevalence and increased mortality risk. Their findings underscored the impact of HIV infection, physiological frailty, and cancer on telomere length and overall health [56].
In May 2024, Hammami and colleagues studied the impact of cigarette smoke on telomere length and associated gene expression. They found that cigarette smoke extract downregulated telomere-stabilizing genes TRF2 and POT1 while increasing the expression of hTERT, a subunit of telomerase linked to cancer, and ISG15, an inflammatory protein. Their research showed that smokers had higher levels of these markers in lung tissue and blood samples, suggesting that smoking accelerates telomere shortening and contributes to inflammation and cancer development [57].
Literature
[1] Kitano, H. Biological Robustness. Nature Reviews Genetics 2004 5:11 2004, 5, 826–837.
[2] Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nature Reviews Molecular Cell Biology 2021 23:1 2021, 23, 74–88.
[3] Lin, J.; Epel, E. Stress and Telomere Shortening: Insights from Cellular Mechanisms. Aging Res Rev 2022, 73, 101507..
[4] López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278.
[5] Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res 1965, 37, 614–636..
[6] Faragher, R.G.A. The Relationship Between Cell Turnover and Tissue Aging. Aging of the Organs and Systems 2003, 1–28.
[7] Callén, E.; Surrallés, J. Telomere Dysfunction in Genome Instability Syndromes. Mutation Research/Reviews in Mutation Research 2004, 567, 85–104.
[8] Reaper, P.M.; di Fagagna, A.; Jackson, S.P. Activation of the DNA Damage Response by Telomere Attrition: A Passage to Cellular Senescence. 2004..
[9] Adwan-Shekhidem, H.; Atzmon, G. The Epigenetic Regulation of Telomere Maintenance in Aging. Epigenetics of Aging and Longevity: Translational Epigenetics vol 4 2018, 119–136..
[10] Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, Health, and Hallmarks of Aging. Cell 2021, 184, 306–322.
[11] Carroll, B.; Korolchuk, V.I. Nutrient Sensing, Growth and Senescence. FEBS J 2018, 285, 1948–1958.
[12] Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and Its Role in the Aging Pathways: Telomere Shortening, Cell Senescence and Mitochondria Dysfunction. Biogerontology 2018 20:1 2018, 20, 1–16.
[13] Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nature Reviews Molecular Cell Biology 2014 15:7 2014.
[14] Campisi, J.; D’Adda Di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nature Rev. Mol. Cell Biol. 2007, 8, 729–740..
[15] Senescence of Mesenchymal Stem Cells (Review) Available online: https://www.spandidos-publications.com/10.3892/ijmm.2017.2912 (accessed on 30 May 2024).
[16] Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The Roles of Senescence and Telomere Shortening in Cardiovascular Disease. Nature Reviews Cardiology 2013 10:5 2013, 10, 274–283.
[17] Sampson, M.J.; Hughes, D.A. Chromosomal Telomere Attrition as a Mechanism for the Increased Risk of Epithelial Cancers and Senescent Phenotypes in Type 2 Diabetes. Diabetologia 2006, 49, 1726–1731.
[18] Levstek, T.; Kozjek, E.; Dolžan, V.; Trebušak Podkrajšek, K. Telomere Attrition in Neurodegenerative Disorders. Front Cell Neurosci 2020, 14, 556488.
[19] Iwasaki, K.; Abarca, C.; Aguayo-Mazzucato, C. Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications. Diabetes Metab J 2023, 47, 441–453.
[20] Lee, Y.H.; Jung, J.H.; Seo, Y.H.; Kim, J.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Association between Shortened Telomere Length and Systemic Lupus Erythematosus: A Meta-Analysis. Lupus 2017, 26, 282–288.
[21] Ball, S.E.; Gibson, F.M.; Rizzo, S.; Tooze, J.A.; Marsh, J.C.W.; Gordon-Smith, E.C. Progressive Telomere Shortening in Aplastic Anemia. Blood 1998, 91, 3582–3592.
[22] Nelson, N.D.; Bertuch, A.A. Dyskeratosis Congenita as a Disorder of Telomere Maintenance. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2012, 730, 43–51.
[23] Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front Cell Dev Biol 2021, 9, 668171.
[24] Wang, Y.; Patell, D.I. Solution Structure of the Human Telomeric Repeat d[AG3(T2AG3)3] G-Tetraplex.
[25] Collins, K.; Mitchell, J.R. Telomerase in the Human Organism. Oncogene 2002, 21, 564–579.
[26] Fernandes, S.G.; Dsouza, R.; Khattar, E. External Environmental Agents Influence Telomere Length and Telomerase Activity by Modulating Internal Cellular Processes: Implications in Human Aging. Environ Toxicol Pharmacol 2021, 85, 103633.
[27] Muoio, D.; Laspata, N.; Fouquerel, E. Functions of ADP-Ribose Transferases in the Maintenance of Telomere Integrity. Cellular and Molecular Life Sciences 2022, 79.
[28] Nagpal, N.; Agarwal, S. Telomerase RNA Processing: Implications for Human Health and Disease. Stem Cells 2020, 38, 1532–1543.
[29] Mir, S.M.; Tehrani, S.S.; Goodarzi, G.; Jamalpoor, Z.; Asadi, J.; Khelghati, N.; Qujeq, D.; Maniati, M. Shelterin Complex at Telomeres: Implications in Ageing. Clin Interv Aging 2020, 15, 827–839.
[30] Kibe, T.; Osawa, G.A.; Keegan, C.E.; Lange, T. de Telomere Protection by TPP1 Is Mediated by POT1a and POT1b. Mol Cell Biol 2010, 30, 1059–1066.
[31] Eliˇ, E.; Janoušková, E.; Janouškov, J.; Janoušková, J.; Ně Casovácasov´casová, I.; Pavloušková, J.; Pavlouškov, P.; Pavloušková, P.; Zimmermann, M.; Hluch´y, M.; et al. Human Rap1 Modulates TRF2 Attraction to Telomeric DNA. Nucleic Acids Res 2015, 43, 2691–2700.
[32] Bianchi, A.; Smith, S.; Chong, L.; Elias, P.; De Lange, T. TRF1 Is a Dimer and Bends Telomeric DNA. EMBO J 1997, 16, 1785–1794.
[33] Khodadadi, E.; Mir, S.M.; Memar, M.Y.; Sadeghi, H.; Kashiri, M.; Faeghiniya, M.; Jamalpoor, Z.; Sheikh Arabi, M. Shelterin Complex at Telomeres: Roles in Cancers. Gene Rep 2021, 23, 101174.
[34] Saretzki, G. Role of Telomeres and Telomerase in Cancer and Aging. International Journal of Molecular Sciences 2023, Vol. 24, Page 9932 2023, 24, 9932.
[35] Pal, D.; Sharma, U.; Singh, S.K.; Kakkar, N.; Prasad, R. Over-Expression of Telomere Binding Factors (TRF1 & TRF2) in Renal Cell Carcinoma and Their Inhibition by Using SiRNA Induce Apoptosis, Reduce Cell Proliferation and Migration Invitro. PLoS One 2015, 10, e0115651.
[36] Wu, Y.; Poulos, R.C.; Reddel, R.R. Role of POT1 in Human Cancer. Cancers 2020, Vol. 12, Page 2739 2020, 12, 2739.
[37] Yang, D.; He, Q.; Kim, H.; Ma, W.; Songyang, Z. TIN2 Protein Dyskeratosis Congenita Missense Mutants Are Defective in Association with Telomerase. Journal of Biological Chemistry 2011, 286, 23022–23030.
[38] Schmutz, I.; Mensenkamp, A.R.; Takai, K.K.; Haadsma, M.; Spruijt, L.; De Voer, R.M.; Choo, S.S.; Lorbeer, F.K.; Van Grinsven, E.J.; Hockemeyer, D.; et al. Tinf2 Is a Haploinsufficient Tumor Suppressor That Limits Telomere Length. Elife 2020, 9, 1–20.
[39] Karlseder, J.; Hoke, K.; Mirzoeva, O.K.; Bakkenist, C.; Kastan, M.B.; Petrini, J.H.J.; De Lange, T. The Telomeric Protein TRF2 Binds the ATM Kinase and Can Inhibit the ATM-Dependent DNA Damage Response. PLoS Biol 2004, 2, e240.
[40] Abbas, T.; Keaton, M.A.; Dutta, A. Genomic Instability in Cancer. Cold Spring Harb Perspect Biol 2013, 5, a012914.
[41] Loayza, D.; De Lange, T. POT1 as a Terminal Transducer of TRF1 Telomere Length Control. Nature 2003 423:6943 2003, 423, 1013–1018.
[42] Li, H.; Cao, Y.; Berndt, M.C.; Funder, J.W.; Liu, J.P. Molecular Interactions between Telomerase and the Tumor Suppressor Protein P53 in Vitro. Oncogene 1999 18:48 1999, 18, 6785–6794.
[43] Artandi, S.E.; Attardi, L.D. Pathways Connecting Telomeres and P53 in Senescence, Apoptosis, and Cancer. Biochem Biophys Res Commun 2005, 331, 881–890.
[44] Wu, K.J.; Grandori, C.; Amacker, M.; Simon-Vermot, N.; Polack, A.; Lingner, J.; Dalla-Favera, R. Direct Activation of TERT Transcription by C-MYC. Nature Genetics 1999 21:2 1999, 21, 220–224.
[45] Lacerte, A.; Korah, J.; Roy, M.; Yang, X.J.; Lemay, S.; Lebrun, J.J. Transforming Growth Factor-β Inhibits Telomerase through SMAD3 and E2F Transcription Factors. Cell Signal 2008, 20, 50–59.
[46] Harada, G.; Neng, Q.; Fujiki, T.; Katakura, Y. Molecular Mechanisms for the P38-Induced Cellular Senescence in Normal Human Fibroblast. The Journal of Biochemistry 2014, 156, 283–290.
[47] Savage, S.A. Telomere Length and Cancer Risk: Finding Goldilocks. Biogerontology 2024, 25, 265–278.
[48] Harley, J.; Santosa, M.M.; Ng, C.Y.; Grinchuk, O. V.; Hor, J.H.; Liang, Y.; Lim, V.J.; Tee, W.W.; Ong, D.S.T.; Ng, S.Y. Telomere Shortening Induces Aging-Associated Phenotypes in HiPSC-Derived Neurons and Astrocytes. Biogerontology 2024, 25, 341–360.
[49] Chebly, A.; Khalil, C.; Kuzyk, A.; Beylot-Barry, M.; Chevret, E. T-Cell Lymphocytes’ Aging Clock: Telomeres, Telomerase and Aging. Biogerontology 2023 25:2 2023, 25, 279–288.
[50] Pepke, M.L. Telomere Length Is Not a Useful Tool for Chronological Age Estimation in Animals. BioEssays 2024, 46, 2300187.
[51] Romero-Haro, A.; Mulder, E.; Haussmann, M.F.; Tschirren, B. The Association between Age and Telomere Length Is Age-Dependent: Evidence for a Threshold Model of Telomere Length Maintenance. J Exp Zool A Ecol Integr Physiol 2024, 341, 338–344.
[52] Abdel-Gabbar, M.; Kordy, M.G.M. Telomere-Based Treatment Strategy of Cardiovascular Diseases: Imagination Comes to Reality. Genome Instability & Disease 2024 5:2 2024, 5, 61–75.
[53] Torigoe, T.H.; Willcox, D.C.; Shimabukuro, M.; Higa, M.; Gerschenson, M.; Andrukhiv, A.; Suzuki, M.; Morris, B.J.; Chen, R.; Gojanovich, G.S.; et al. Novel Protective Effect of the FOXO3 Longevity Genotype on Mechanisms of Cellular Aging in Okinawans. npj Aging 2024 10:1 2024, 10, 1–9.
[54] Karimian, K.; Groot, A.; Huso, V.; Kahidi, R.; Tan, K.T.; Sholes, S.; Keener, R.; McDyer, J.F.; Alder, J.K.; Li, H.; et al. Human Telomere Length Is Chromosome End-Specific and Conserved across Individuals. Science (1979) 2024, 384, 533–539.
[55] Mason, C.E.; Sierra, M.A.; Feng, H.J.; Bailey, S.M. Telomeres and Aging: On and off the Planet! Biogerontology 2024 25:2 2024, 25, 313–327.
[56] Liang, X.; Aouizerat, B.E.; So-Armah, K.; Cohen, M.H.; Marconi, V.C.; Xu, K.; Justice, A.C. DNA Methylation-Based Telomere Length Is Associated with HIV Infection, Physical Frailty, Cancer, and All-Cause Mortality. Aging Cell 2024, 00, e14174.
[57] Hammami, S.; Lizard, G.; Vejux, A.; Deb, S.; Berei, J.; Miliavski, E.; Khan, M.J.; Broder, T.J.; Akurugo, T.A.; Lund, C.; et al. The Effects of Smoking on Telomere Length, Induction of Oncogenic Stress, and Chronic Inflammatory Responses Leading to Aging. Cells 2024, Vol. 13, Page 884 2024, 13, 884.