Bryan Johnson is an enigma in the longevity space, someone who is difficult to place in a familiar category or determine the net impact of.
Johnson is a successful tech entrepreneur of humble origins who sold his company Braintree Venmo to PayPal in 2013 for 800 million dollars. However, years of hard work had taken a toll on him. Johnson recalls being frequently depressed and dissatisfied with his own health and body image. A couple of years ago, he decided to take matters into his own hands – in quite a radical manner.
Johnson became interested in age reversal and eventually hired a team of physicians to help him develop the most effective age-reversing protocol possible, regardless of the amount of money or effort required. He called it Blueprint. Several months later, Johnson published the preliminary results, crowning himself the world champion in age reversal. This, predictably, caused quite a splash and a rush to explain this phenomenon.
Blueprint can be seen as an advanced form of biohacking that requires not just tons of money – some biohackers have such financial resources too – but also jaw-dropping levels of dedication that seem almost impossible to replicate.
The stated goal of this venture was to slow the aging of a single person to the maximum extent possible with existing anti-aging interventions. Of course, as we have been clear about, there aren’t many of those around, and this is why the famed Blueprint consists mostly of daily exercise, a strict plant-based diet with intermittent fasting, optimized sleep, piles of supplements, and various skin treatments.
However, the bulk of the 2-million-dollar-a-year budget goes not to the treatments and supplements but to hundreds of tests. The dedicated team of physicians regularly monitors about 70 organs in Johnson’s body. This includes not just comprehensive blood tests that go far beyond regular bloodwork, but also ultrasound, MRI, and more exotic stuff. All this happens in-house, in a room chock-full of expensive medical equipment inside Johnson’s Los Angeles residence. Of course, testing is important not just for measuring the effects of the interventions but also for early diagnostics.
Based on a daily routine
There is a dedicated website that shows all the details of Blueprint, but here are the basics.
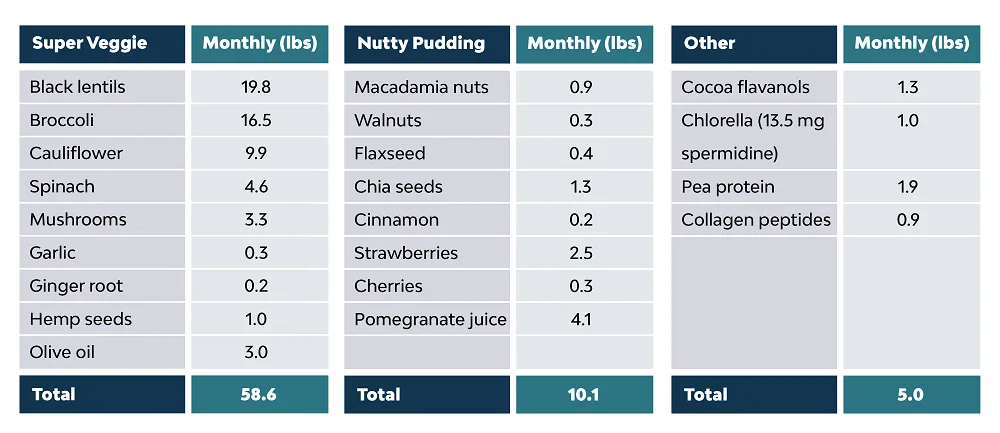
Blueprint is built on a strict daily routine. Johnson wakes up naturally between 4:30 and 5:30 am, drinks a smoothie he calls the Green Giant, which contains goodies such as spermidine, flavanols, and collagen peptides, swallows literally handfuls of pills, then hits the gym for about an hour.

When the morning skincare routine is done, Johnson eats his first meal. It’s always the same, just like the second one. The former is called Super Veggie, and the latter Nutty Pudding. Johnson allows himself to get a bit creative only with the third meal, but the amount of nutrients and calories always stays the same.
He consumes exactly 1977 calories a day, which is his year of birth that just happened to be close enough. Johnson says he’s on 25% caloric restriction versus the recommended daily amount of 2500 calories for his activity levels. Johnson is a vegan by choice, and the only animal-based product he consumes is collagen peptides.
Johnson practices intermittent fasting, having the last meal of the day at around 11 AM. The fasting period lasts 16-18 hours. Johnson tried a one-meal-a-day (OMAD) regimen, but it resulted in his body fat dropping to 3%, which he considered too low. Currently, it’s 5%, on par with elite athletes. Johnson admits being constantly hungry, but he learned to enjoy the feeling. He also enjoys his food quite a lot.
Johnson is very serious about his sleep quality, which is constantly monitored. He takes melatonin, wears blue light-blocking glasses for an hour before going to bed (always at 8:30 PM), and sleeps alone in a pitch-black bedroom on a temperature-controlled mattress. He says that caloric restriction and good sleep are probably Blueprint’s most effective components.
Johnson told me that he tried meditation for some time, but couldn’t measure its effects, so he switched to breathing exercises that increased his heart rate variability (HRV).
Johnson’s skincare routine is quite elaborate. He uses several creams and undergoes laser treatments. At various times, the list included also things like acid peels and fat injections. Although skin aging is important to address for health reasons, Johnson is probably after good looks too, as he also dyes his hair.
The results, at least in appearance, are quite impressive. Johnson is ripped, while still looking light and nimble. His smooth, pale skin belies his chronological age, although some subtle facial features still hinted at it as we were chatting on Zoom. This created an eerie cognitive dissonance that dissipated after a few minutes.
Running the numbers
Johnson calls himself “potentially the most measured person in human history,” and so the ultimate proof is in the many, many numbers.
The several tests that Johnson undergoes a day are all longevity-oriented. Naturally, they include epigenetic clocks, such as DunedinPACE, which focuses on the rate of aging rather than estimating a chrological age. The Blueprint website uses these numbers to show the rapid age reversal that Johnson underwent during the program’s first months. While being 45 chronologically and somewhat older epigenetically at baseline, he says he was able to roll his epigenetic age about four years back.
According to DunedinPACE, Johnson’s rate of aging was 0.76, although a December 2022 update puts it at an even more impressive 0.69. Johnson jokes that, compared to an average person, he “gets October, November, and December for free.”
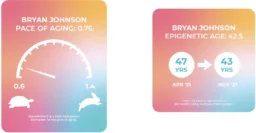
The small print says that when calculating his epigenetic age, Johnson used a multi-clock average. In a blog post that boasts his “epigenetic age reduction world record”, Johnson offers a fuller picture:
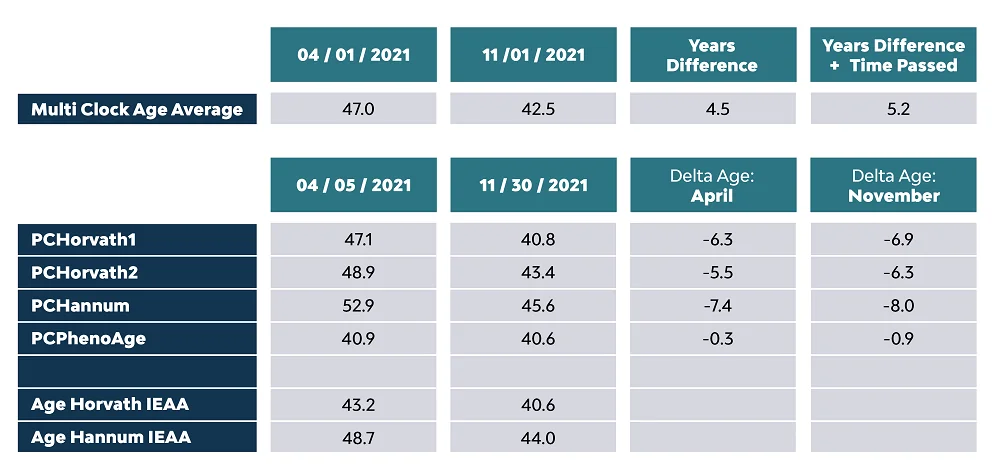
While epigenetic clocks have become a popular biomarker of aging, they are not without their drawbacks, and Johnson himself calls them “the silver standard” for measuring biological age. Horvath1 and Hannum are first-generation clocks developed in 2012-2013, while Horvath2 is the newer Horvath skin and blood clock. PC refers to a principal component analysis, a technique used to reduce the clocks’ variability.
The second-generation clock PhenoAge, which is widely relied upon in research, shows both lower biological age at baseline and a much more modest reversal. IEAA stands for “intrinsic epigenetic age acceleration”, which means that the results were corrected for age-related changes in blood cell composition that accompany immune system aging to supposedly better reflect fundamental aging processes.
Dr. Steve Horvath, the “father of epigenetic clocks” who is currently a principal investigator at Altos Labs, says he found the reduction in the readings of the first-generation clocks “very impressive.” He notes however that “first-generation clocks are less predictive of mortality and morbidity risk than second-generation clocks which were designed for that task.”
Horvath told me that he offered a couple of times to reanalyze Bryan’s team data, albeit indirectly, in his correspondence with Vice. The outlet reported that Johnson’s team declined the offer.
Horvath also met Johnson in person in February at the Academy for Health & Lifespan Research meeting. “On that day, my nonprofit Epigenetic Clock Development foundation offered free epigenetic clock tests to all participants,” he recounts. “While (Johnson) was presenting at the podium, I also explicitly asked him to participate in that free GrimAge test. He declined. I don’t know why since this was an opportunity for an unbiased evaluation by an independent group, and several articles show that GrimAge is the most accurate epigenetic clock when it comes to predicting mortality and morbidity risk.”
In the same blog post, Johnson reports that GrimAge was used alongside other clocks, but “has not yet been calculated from this dataset and will be soon.” However, I could not find any subsequent updates.
Potential disagreements aside, Horvath says he “greatly admires Johnson’s discipline and dedication.”
Measuring biological age is a hard and novel task, and there are few established protocols for it, which is unsurprising considering that scientists still disagree about what aging is. In addition to the epigenetic clocks, Johnson’s team measures a great many biomarkers, comparing them to average values for age categories. For example, when Johnson says that his heart’s biological age plummeted from 60 to 37 years, he mostly means that he was able to increase his maximum heart rate from 169 to 183, which are associated with those respective ages. In a similar way, Johnson’s team demonstrates “age reduction” in many of his organs.

Just how impressive is this? It’s hard to tell. First, there’s a lot of variability in the age categories. Johson is a very healthy 45-year-old, and comparing him to an average 25-year-old might seem a bit unfair.
Even more importantly, human mortality risk increases exponentially, which is known as the Gompertz–Makeham law. This is probably because mechanisms that prevent accumulation of damage slowly get dysregulated themselves, and damage becomes a runaway train.
This dramatic and unfortunate turn of events starts somewhere around Johnson’s (and mine) age, which is why age-related diseases are, well, age-related. Some animals, such as the naked mole rat, have evolved so-called negligeable senescence, meaning that their mortality risk barely increases with time, but humans can only dream of such superpowers.
The obvious implication is that there is much less difference in terms of health between a 45-year-old and a 25-year-old than between a 65-year-old and a 45-year-old. Currently, Johnson’s highly demanding protocol (“an almost full-time job”, he says) seems to be working well, but soon, it may become a very uphill battle.
Just how rigorous?
One important claim that Johnson reiterates a lot is that Blueprint is “scientifically rigorous”. However, this requires explanation.
“Rigorous” does not equal “proven”. We do have strong evidence that links longevity to exercise, healthy diet, and maybe good sleep, although there is some debate about the optimal amount of physical activity and sleep or which diet is the healthiest. However, for many of the drugs and supplements on Johnson’s menu, the evidence is much shakier and does not account for possible interactions between them, which are mostly unresearched.
Given all this complexity, how did Johnson arrive at his current regimen? Basically, a conclave of physicians sifts through existing scientific evidence (more than 1,000 articles and counting) and throws whatever seems to fit the efficacy/safety profile into the mix. However, given that the evidence is sometimes fragmentary and even contradictory, surely debates must be relentless?
“The amount of data and the frequency it’s captured at substantially helps the debate,” Johnson explained to me. “Like the Bible can support multiple religions claiming they’re the true Church of God, health and wellness supports a similar number of people claiming they’re the true God of health and wellness, and the only way a conversation moves forward productively is when it can be baselined in data. This is why we try to get as much data as we can.” As Johnson comes from a Mormon family, the choice of analogy is probably not an accident.
“If there’s a situation in the room when the team disagrees on something, how is it handled?” I asked.
“It’s just constructive dispute resolution, constructive problem solving,” Johnson replied. “No one is storming out of the room, nobody’s raising their voice, no one’s getting emotionally upset. Everything’s calm, deliberate, and respectful. It’s just the typical process of trying to reconcile different opinions.”
Two major differences separate Blueprint from clinical trials. In trials, there is usually a single intervention and many participants. In Blueprint, it’s the exact opposite. So, I asked him how it’s possible to know what’s working and to what extent.
Johnson said, albeit without offering much detail: “The measurement protocol we have is sufficiently robust that we can look at the effect these things are having. I think it’s mostly true that unless we can measure something, we don’t do it. We do dozens of measurements, and we can typically map the data back to the intervention. It’s not the controlled environment where you isolate one variable, because we are choosing to move faster.”
For a while, I kept asking him about the rationales for various interventions, eliciting similar responses. How did they choose the specific intermittent fasting protocol or the level of caloric restriction? “I suppose I’ll give you the same answer,” Johnson smiled. “We’re looking at hundreds of markers, and each one has a certain relationship to the intervention.”
So, I tried to offer my interpretation: “Basically, it’s ‘do no harm’, right? If you see that your readings improve or stay the same, you don’t change direction?”
“That’s right”, he nodded, “and currently, everything is pointing in a positive direction”.
Still, this setup makes some experts raise their eyebrows. Dr. Morgan Levine, formerly at Yale and now working for Altos Labs, told Vice that “it sounds like they’re measuring a thousand things at once.” According to Levine, with that many interventions, it’s extremely hard to deduce causal links.
The debatable value of n=1
Being an n=1 experiment (that is, having only one participant), it’s not immediately obvious how Blueprint can help longevity research. What insights can scientists glean from it? Johnson says it’s just “a different way of trying to solve the same problem.”
There are several things that people can observe about this. Just like you’re saying, a person may observe that we’re doing too many things at a time to isolate effect, or that n=1 is an insufficient study design to create reliable outcomes, and so on. These are all legitimate observations that we acknowledge.
Recently, I attended a gathering of 25 of the world’s leading anti-aging researchers. Many expressed frustration to me at how slowly their research was moving, because of the constraints on their ability to do things, specifically multi-variable interventions. So, I think, there’s a decent number of people who see the shortcomings and limitations of what we’re doing but also appreciate it because it accomplishes things that they can’t do in their research.
Johnson has a point here. Prof. George Church of the Harvard Medical School, one of the most famed geroscientists, says Johnson is doing important work. “The reason I think it’s important”, Church explained, “is that it can help to work out the details of obtaining, reporting, and sharing baseline multi-modal and multi-omic data.” Basically, it’s not just what data Johnson’s team collects, but also how it does it that can inform future trials.
The n=1 issue doesn’t bother Church. He reminded me that self-experimentation has always been a part of science. In 2019, Church even co-authored a paper on the history and ethics of self-experimentation. “Barry Marshall’s experiment was n=1”, he said, referring to the Australian Nobel laureate who, in 1984, infected himself with H. pilory to prove that this bacterium was a major cause of ulcers.
“My main critique”, added Church, “is that I prefer randomized crossover clinical trials even at the n=1 stage, although I can see how that may be challenging in a constantly updating prototype environment.” In other words, n=1 studies can be made more rigorous and scientifically valuable, but this would probably interfere with Johnson’s primary goal of minimizing his pace of aging.
Dr. Aubrey de Grey, a well-known longevity research veteran and head of the Longevity Escape Velocity foundation, concurs: “All self-experimentation has the n=1 problem, but that’s not binary: we can still glean information by examining results from such people, even if the interpretation is weaker than in randomized controlled trials.”
Longevity for the masses
I asked Johnson whether he was interested in fighting aging on a wider, global scale.
“Yes, this is why I made everything available for free,” he confirmed, referring to his protocol.
I then asked him if he has considered more ways to contribute to the fight against aging, such as funding longevity research.
This is a question about resource allocation. Is the available money spent best being in the government’s hands, where it decides which labs get the money? Or is it better in the hands of pharmaceuticals? This is a larger societal question – how to allocate resources to achieve the end goal of health and wellness.
In my case, because I’ve made money myself, it sits within my discretion. I started a venture fund where I spent a hundred million dollars investing in deep tech, synthetic biology, nanotechnology, genetics, and therapeutics.
I think what we’re doing at Blueprint has had an interesting impact on the world, bringing attention to the field of anti-aging. It has had a positive effect on many people in their contemplation of what life decisions they’re making. Everyone’s going to disagree on who’s best served to have money and for what objective. This is my best guess on how to achieve the most substantial impact.
It is true that Johnson has attracted a lot of attention, but not necessarily of a good kind. He’s been dragged through the mud in the media for being “a rich guy trying to live forever” – a harmful stereotype that the longevity community has been fighting for years.
“I love the haters,” Johnson responded with a smile. “I love all of it, and I think it’s a healthy societal conversation. We have built our society on addictions – to food, to digital media, social media, gaming, porn. We are walking addictions, and all corporate powers in the world are striving to make each of us addicted to their thing.”
In Johnson’s mind, despite all his wealth and business interests, he’s not part of the corporate world but a champion of the individual who’s been duped by this world into spending hard-earned money on self-destruction.
I think what (the critics) are really commenting on is the way we’ve built society that is pretty tough for an individual. Practically, on the way to work, a person needs to pass 20 fast food joints, 20 places that sell sugary drink as a morning wake-up beverage. Then you have to navigate the addiction to social media all day, then binge watching at night while eating the wrong foods. It’s pretty unfair to the individual that we then ask them to be in a good, stable health place, without giving them proper healthcare.
De Grey is supportive again: “We shouldn’t get hung up on the amount that Bryan is spending on Blueprint, nor should we castigate him for not supporting the longevity mission in other ways. If I’ve learned anything over the years in my interactions with HNWIs (high net worth individuals), it’s that they are all different and they know their own minds. We should leverage what they already want to do. The stereotype of HNWIs being selfish in wanting to live forever is a figment of the general public’s imagination, constructed only to put out of their minds the fact that they want not to age just as much as the HNWIs do.”
Johnson insists that he’s showing the way to the masses and not just spending millions on his personal immortality quest. “The expectation I have for Blueprint is not that everyone’s going to do it,” he explained. “It’s really difficult to do. It costs a lot of money to have all this measurement infrastructure and to go through the science, which is why I made it free for everybody.”
So, instead of following Blueprint to the letter, he noted that “a person in the US can spend $1500 a month, including groceries, to do the basics. Wouldn’t it be interesting, helpful, and cool if we all did this by default? If it was the norm, and we didn’t celebrate self-destructive behaviors?”
An appetite for self-destruction
The motif of resisting self-destructive behavior runs deep in Johnson’s ideology. Moreover, you cannot really understand Blueprint without considering Johnson’s personal story and struggles. He grew up in a family where unhealthy food habits were the norm. Despite his attempts to stay in shape, by his mid-thirties, he was feeling controlled by what he dubbed “the Evening Bryan” – the unruly part of his personality that lured him into binge-eating before bed and other self-destructive actions, “ruining life for all other Bryans”.
At some point, Johnson “playfully revoked Evening Bryan’s decision-making authority to eat food”. Evening Bryan put up quite a fight, but eventually, Johnson was able to relegate him to the dustbin of history. This developed into a deeply held belief and merged with the rest of Johnson’s philosophy.
Johnson understood that he didn’t want his brain to decide what to eat, but rather his body as a whole. How can you know what your body is telling you? By measuring it. Your body will decide what’s good for it, and you should blindly follow its guidance.
What I’m proposing with Blueprint is to try building an algorithm based upon measurements and scientific evidence that cares for me better than I can myself. It’s just like when I fly a plane, I know the autopilot is going to do it better than I’m able to.
Today, Johnson says that he feels liberated by not having to decide what to eat, which also saves time. He’s quite happy with his routine, although his zealotry in following it might feel off-putting to some. But maybe he has no choice: the impulses are still there, lurking in the darkness. When asked whether he drinks coffee, he said he doesn’t, because for him, coffee is an escalation drug. “Every time I’ve tried to drink coffee, it becomes a runaway train,” he explained. “I drink green tea because I enjoy it, for the health properties, and because it’s stable for me.”
Johnson goes to great lengths to never deviate from his routine, even when it requires him to cut short a conversation with his children to go to bed. “I’ve found that if I give myself the slightest opening of choice, I will make the wrong decisions every single time,” he says.
But is relinquishing any choice the only choice for everyone, or just for people who might have trouble controlling their impulses? As someone who fought himself for months when switching to intermittent fasting or giving up meat, I understand the struggle and the rationale, and yet, this feels way too extreme.
“I do get the concept of self-destructive behaviors,” I told him, “but is consciously trading a minuscule increase in the risk of disease or death for some amount of pleasure necessarily self-destructive?”
Johnson got philosophical:
We’re at this special moment in time when AI is moving at the speed it is, with sustainability of our planet in question, and humans dangerously at each other’s throats. It’s probably a good time for us to seriously consider our next evolutionary step, and as a baby step in that process, we should consider these self-destructive behaviors to be ridiculous. We’re in a different era than ever before, and I don’t think we’ve fully reconciled with the specialness and seriousness of this moment.
“I still don’t get how going out with friends or having a bottle of beer once in a while can be ridiculous, self-destructive, or a threat to our species’ survival”, I noted. He replied:
I talk about self-destructive behaviors very generally, and if you want to get into details, that’s a wonderful discussion. There’s a good concept called micromorts where you can mathematically quantify what is self-destructive and to what extent. What I’m suggesting is a general idea that doing things that accelerate aging, decay, disability is generally not a good idea.
Even though they’re a norm today, even if we think they give us happiness, we should take a breath and ask ourselves, is it possible that in a decade, or two, or three, our future selves will look back at us and say, ‘can you believe the silliness of what those people did in their behaviors? They told all those pretty stories to cover up the fact that they were doing self-destructive things.’ What I’m saying is that everything about change is uncomfortable.

A rejuvenation Olympian
It is hard to estimate the size of Blueprint’s following, and there is no formal community for people who have taken it up. There is, however, a website called Rejuvenation Olympics created by Johnson’s team in collaboration with TruDiagnostic, an epigenetic test kit manufacturer. It says that over 20 thousand people have taken the test, and 1,750 have done it more than once – that is, measured their epigenetic age longitudinally. The website has a leaderboard, with two “rejuvenation athletes” showing results that are pretty close to Johnson’s who’s in the first place.
Johnson is indeed a top athlete in his chosen field, and he does what top athletes do: put enormous efforts into achieving maximum possible gains. Of course, it’s not for everyone. Moreover, it’s still not clear how healthy pushing yourself to the limit in pursuit of age reversal is in the long run.
Dr. Brad Stanfield, a primary care physician running his own popular YouTube channel focused on health and preventative medicine, strikes a sobering tone: “There are numerous evidence-based strategies that people can use to prevent disease and improve their energy levels, all of which are part of the current clinical guidelines that primary care physicians, such as myself, use. While everyone has the option to care for their health in any way they see fit, it’s important to note that Bryan’s protocol deviates significantly from the current preventative care clinical guidelines and may cause more harm than benefit.”
However, Blueprint’s core tenets – good diet, good sleep, exercise – are largely based on common sense, and Johnson is correct in identifying certain consumption and behavioral patterns as destructive. There is indeed a lot of self-harming going on, and people who are reasonably careful don’t have to be top athletes or multimillionaires to turn things around.
What’s next for Johnson? He has indicated his general readiness to try more drastic anti-aging treatments, such as gene therapies, as they appear. This means we will probably be hearing a lot from him. To quote de Grey, “more power to Bryan, and let’s hope that his self-experimentation gives us information that no one else does.”
We would like to ask you a small favor. We are a non-profit foundation, and unlike some other organizations, we have no shareholders and no products to sell you. All our news and educational content is free for everyone to read, but it does mean that we rely on the help of people like you. Every contribution, no matter if it’s big or small, supports independent journalism and sustains our future.