As the second month of spring, April is often associated with rebirth, and we’ve added rejuvenation to the mix with cryptocurrency donation initiatives, biotechnology company progress, and plenty of science.
LEAF News
 A Busy Spring for Rejuvenation Research and Advocacy: Lifespan.io’s birthday was in April, and we proudly celebrated nine years of being a non-profit organization that advocates, educates, and fundraises for healthy life extension.
A Busy Spring for Rejuvenation Research and Advocacy: Lifespan.io’s birthday was in April, and we proudly celebrated nine years of being a non-profit organization that advocates, educates, and fundraises for healthy life extension.
An Opportunity to Support Aging Research with Gitcoin: We have two scientific research projects in the new Gitcoin fundraising round. Help us to combat Alzheimer’s disease or improve how clinical trials are conducted today! Gitcoin is a decentralized science (DeSci) platform that utilizes blockchain technology to support the development of open-source projects using Web3 technologies.
Team and activities
 Cardio Diagnostics Announces Strategic Engagement With Us: Cardio Diagnostics Holdings, Inc (Nasdaq: CDIO), an artificial intelligence-powered precision cardiovascular medicine company, has announced a strategic engagement with Lifespan.io.
Cardio Diagnostics Announces Strategic Engagement With Us: Cardio Diagnostics Holdings, Inc (Nasdaq: CDIO), an artificial intelligence-powered precision cardiovascular medicine company, has announced a strategic engagement with Lifespan.io.
Rejuvenation Roundup Podcast
Ryan O’Shea of Future Grind hosts this month’s podcast, showcasing the events and research discussed here.
Journal Club
Reducing DNA Damage With DREAM: In Journal Club this month, we explored a new study published in Nature Structural and Molecular Biology, where researchers demonstrated that by manipulating the DREAM protein complex, a major regulator of DNA damage response, it may be possible to reduce the number of DNA mutations accumulated with age.
Advocacy and Analysis
Bryan Johnson’s Race Against Time: Bryan Johnson is an enigma in the longevity space, someone who is difficult to place in a familiar category or determine the net impact of. Johnson is a successful tech entrepreneur of humble origins who sold his company Braintree Venmo to PayPal in 2013 for 800 million dollars.
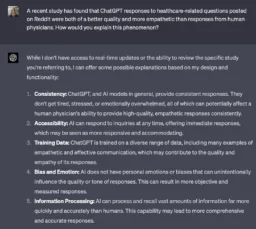
 What AI Technology Is Doing for Longevity Now: In March 2023, MIT Technology Review revealed that Sam Altman, the CEO of OpenAI (ChatGPT), was the mystery investor behind the $180 million investment into stealth startup Retro Biosciences, a biotech company with the ambition of “adding 10 years to the human lifespan.”
What AI Technology Is Doing for Longevity Now: In March 2023, MIT Technology Review revealed that Sam Altman, the CEO of OpenAI (ChatGPT), was the mystery investor behind the $180 million investment into stealth startup Retro Biosciences, a biotech company with the ambition of “adding 10 years to the human lifespan.”
Research Roundup
 Cold Temperatures Stimulate Lifespan-Associated Protein: A paper published in Nature Aging describes how cold temperatures stimulate the production of PA28γ, a protein that appears to increase lifespan in worms and cells.
Cold Temperatures Stimulate Lifespan-Associated Protein: A paper published in Nature Aging describes how cold temperatures stimulate the production of PA28γ, a protein that appears to increase lifespan in worms and cells.
Young Microbiomes in Very Old People: Research published in Nature Aging has illustrated how the gut microbiomes of the longest-lived people are more likely to have bacterial populations associated with youth.
 Low Carb Intake Linked to Insulin Resistance: Scientists have published a new study suggesting that low carbohydrate consumption is significantly associated with increased insulin resistance in healthy, lean people.
Low Carb Intake Linked to Insulin Resistance: Scientists have published a new study suggesting that low carbohydrate consumption is significantly associated with increased insulin resistance in healthy, lean people.
Reducing Axonal Death and Inflammation in Mouse Brains: Researchers have published a study in Aging Cell on how inhibiting the death of axons in the brain protects the brains of old mice from inflammation.
 Air Pollution May Drive Lung Cancer via Inflammation: Researchers have concluded that airborne fine particulate matter, which has been consistently linked to cancer, promotes lung cancer via inflammation and not necessarily via mutagenesis.
Air Pollution May Drive Lung Cancer via Inflammation: Researchers have concluded that airborne fine particulate matter, which has been consistently linked to cancer, promotes lung cancer via inflammation and not necessarily via mutagenesis.
Physical Activity, Sleeping, Sedentary Behavior, and Aging: Regular exercise, getting enough sleep, and avoiding sedentary behavior are frequently reported as being important in determining how slowly we age. A team of researchers recently set out to determine the link.
 Examining a Factor in the Diabetic Heart: Research published in Heliyon has outlined the effects of F-Klb, a signaling molecule receptor that is released under metabolic stress, on the hearts of diabetic patients. It is the receptor for fibroblast growth factor 21 (FGF21), a regulator of metabolism that is produced both by adipose (fat) cells and the liver.
Examining a Factor in the Diabetic Heart: Research published in Heliyon has outlined the effects of F-Klb, a signaling molecule receptor that is released under metabolic stress, on the hearts of diabetic patients. It is the receptor for fibroblast growth factor 21 (FGF21), a regulator of metabolism that is produced both by adipose (fat) cells and the liver.
Age-Related Changes in RNA Transcription Speed: Research published in Nature has described transcriptional elongation changes in the cell with age and how they may be linked to lifespan. Transcriptional elongation is a fundamental biological process that affects the basic steps involved in the production of RNA, which is responsible for executing DNA instructions.
 Comparing Mushroom and Animal Protein for Muscle Building: Scientists have reported that protein derived from mushrooms (mycoprotein) has a similar impact on muscle mass and strength as animal-based protein in young, healthy people undergoing resistance training.
Comparing Mushroom and Animal Protein for Muscle Building: Scientists have reported that protein derived from mushrooms (mycoprotein) has a similar impact on muscle mass and strength as animal-based protein in young, healthy people undergoing resistance training.
Review: Moderate Drinking Doesn’t Lower Mortality: A new comprehensive meta-analysis failed to find any protective effect of moderate drinking on mortality risk. There is an ancient idea that while excessive drinking will kill you, moderate alcohol consumption is actually good for you, and until recently, it seemed to be supported by scientific evidence.
 Heart Organoids May Change Future Research: Researchers publishing in Nature Biotechnology have generated epicardioids, which are pluripotent, self-organizing stem cells that allow for better understanding, research, and potentially prevention and treatment of heart disease. The epicardium, a layer of cells surrounding the heart, plays a major role in human embryonic development.
Heart Organoids May Change Future Research: Researchers publishing in Nature Biotechnology have generated epicardioids, which are pluripotent, self-organizing stem cells that allow for better understanding, research, and potentially prevention and treatment of heart disease. The epicardium, a layer of cells surrounding the heart, plays a major role in human embryonic development.
Rapamycin Rescues Age-Impaired Blood Flow in Mice: Scientists have concluded that rapamycin treatment started in early mid-life can prevent age-related blood flow impairment, peripheral artery disease, in the hindlimbs of wild-type, atherosclerotic, and Alzheimer’s model mice.
 Amyloid-β Clearance by the Liver Might Help with Alzheimer’s: Chinese scientists have found that the liver removes amyloid-β from circulation in mice, which also decreases its levels in the brain. The age-related impairment of this process might offer a new clue for fighting Alzheimer’s disease.
Amyloid-β Clearance by the Liver Might Help with Alzheimer’s: Chinese scientists have found that the liver removes amyloid-β from circulation in mice, which also decreases its levels in the brain. The age-related impairment of this process might offer a new clue for fighting Alzheimer’s disease.
Case Reports of Sclerotic Fibrosis in the Heart: A pair of case reports published in Heliyon have shed more light on the connection between systemic sclerosis and fatal heart failure, highlighting a need for early diagnosis and treatment.
 Uncovering a Predictive Biomarker for Alzheimer’s: Researchers publishing in Alzheimer’s and Dementia have published a correlation between the Alzheimer’s-linked protein tau and another protein, bisecting N-acetylglucosamine (GlcNAc), which suggests its usefulness as a biomarker.
Uncovering a Predictive Biomarker for Alzheimer’s: Researchers publishing in Alzheimer’s and Dementia have published a correlation between the Alzheimer’s-linked protein tau and another protein, bisecting N-acetylglucosamine (GlcNAc), which suggests its usefulness as a biomarker.
Sleep Quality and Duration Associated with Stroke: Showing yet again that sleep is a serious matter, scientists have reported that short sleep duration, snoring, and long naps are linked to a significantly elevated risk of acute stroke.
 Increased Energy Efficiency Might Drive Weight Regain: Scientists have discovered that formerly obese mice that became leaner have greatly improved energy efficiency, which might be preventing their complete return to normal weight.
Increased Energy Efficiency Might Drive Weight Regain: Scientists have discovered that formerly obese mice that became leaner have greatly improved energy efficiency, which might be preventing their complete return to normal weight.
Examining Cellular Stemness as a Tissue Attribute: In a preprint paper published on bioRxiv, researchers including João Pedro de Magalhães have gotten a glimpse at how stemness declines across tissues with aging.
 Reprogramming Fibroblasts in Vivo for Heart Repair: Scientists from Duke University have found a way to make adult fibroblasts differentiate into cardiomyocytes, which might help develop better heart attack treatments.
Reprogramming Fibroblasts in Vivo for Heart Repair: Scientists from Duke University have found a way to make adult fibroblasts differentiate into cardiomyocytes, which might help develop better heart attack treatments.
Simply Being Overweight May Not Be Harmful in Aging: According to a new large-scale populational study, being overweight is not associated with either significant risks or benefits between the ages of 45 and 85. Obesity, however, is a clear risk factor.
 Hair Pigment Stem Cells May Work Differently: Researchers publishing in Nature have made the surprising discovery that pigmentation cells (melanocytes) can naturally transition back into the stem cell state in a process called dedifferentiation.
Hair Pigment Stem Cells May Work Differently: Researchers publishing in Nature have made the surprising discovery that pigmentation cells (melanocytes) can naturally transition back into the stem cell state in a process called dedifferentiation.
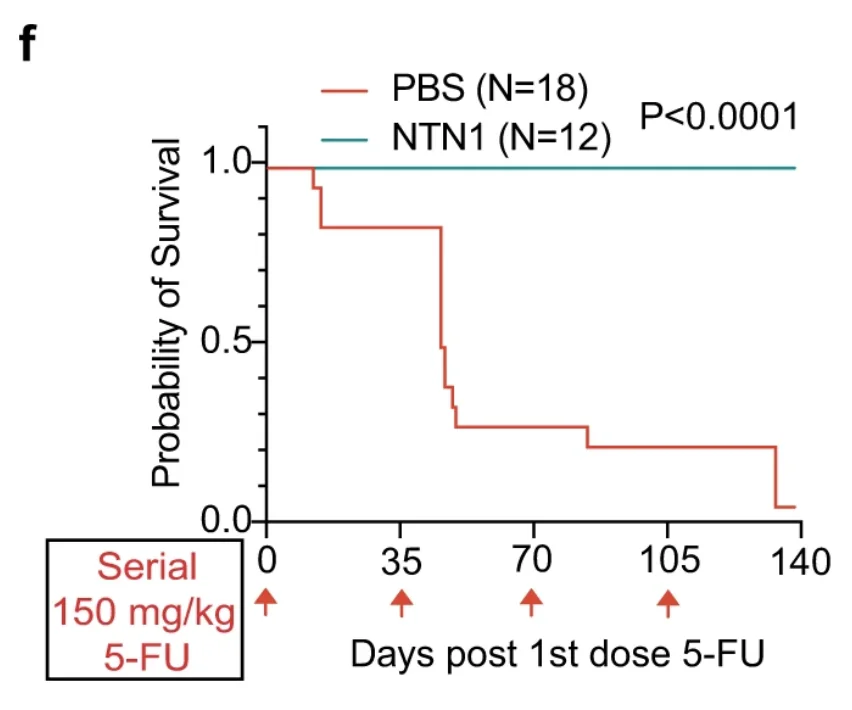
Netrin-1 Rescues Blood Stem Cells in Mice: Scientists have discovered that the protein Netrin-1 alleviates the age-related decline in hematopoietic stem cell function in mice, enhancing HSC transplantation and protecting these mice from the harmful effects of chemotherapy.
Ketone bodies: A double-edged sword for mammalian life span: Endogenous ketogenesis affects mammalian survival, and ketone body supplementation may represent a double-edged sword with respect to survival.
High-Intensity interval training reduces transcriptomic age: A randomized controlled trial: A low dose of HIIT can reduce an mRNA-based measure of biological age in sedentary adults between the ages of 40 and 65 years old. Other changes in gene expression were relatively modest.
Physical activity is associated with slower epigenetic ageing—Findings from the Rhineland study: These findings suggest that regular physical activity slows epigenetic aging by counteracting immunosenescence and lowering cardiovascular risk.
High-Dose Spermidine Supplementation Does Not Increase Spermidine Levels in Blood Plasma and Saliva of Healthy Adults: This study’s results suggest that dietary spermidine is presystemically converted into spermine, which then enters systemic circulation.
Activation of telomerase by TA-65 enhances immunity and reduces inflammation post myocardial infarction: TA-65 increased all major lymphocyte subsets and reduced C-reactive protein in elderly patients 12 months after a heart attack.
Senolytics rejuvenate the reparative activity of human cardiomyocytes and endothelial cells: These results open the path to further studies on using senolytic therapy in age-related cardiac deterioration and rejuvenation.
Engineering longevity—design of a synthetic gene oscillator to slow cellular aging: These results establish a connection between gene network architecture and cellular longevity that could lead to rationally designed gene circuits that slow aging.
AI-Predicted mTOR Inhibitor Reduces Cancer Cell Proliferation and Extends the Lifespan of C. elegans: TKA001 inhibits human cancer cell proliferation in vitro and extends the lifespan of Caenorhabditis elegans, suggesting that TKA001 is able to slow aging in vivo.
Longitudinal associations between use of antihypertensive, antidiabetic, and lipid-lowering medications and biological aging: Calcium channel blockers may decrease biological aging as measured by epigenetic and functional biomarkers.
Vitamin D Supplementation and Its Impact on Mortality and Cardiovascular Outcomes: It appears to lower the risk of all-cause mortality while not showing a decrease in specific cardiovascular morbidity and mortality risk.
News Nuggets
Longevity Prize Announces First Winners: The winners of the Hypothesis Prize have been announced as part of the Longevity Prize initiative. This is an important step for funding rejuvenation research and sets a great precedent for future longevity-focused prizes and open science.
 The Maximon Longevity Prize 2023: 50,000 CHF (56,000 USD): Maximon, Europe’s first longevity company builder, is pleased to announce that applications for the Maximon Longevity Prize 2023 are now open. This prestigious prize is awarded to translational longevity research projects that demonstrate exceptional potential to extend human healthspan and lifespan.
The Maximon Longevity Prize 2023: 50,000 CHF (56,000 USD): Maximon, Europe’s first longevity company builder, is pleased to announce that applications for the Maximon Longevity Prize 2023 are now open. This prestigious prize is awarded to translational longevity research projects that demonstrate exceptional potential to extend human healthspan and lifespan.
Life Biosciences Claims Visual Restoration in Primates: Life Biosciences, a company co-founded by the well-known Dr. David Sinclair, has recently claimed that it has reversed a form of neuropathy in nonhuman primates through gene therapy and epigenetic reprogramming.
We would like to ask you a small favor. We are a non-profit foundation, and unlike some other organizations, we have no shareholders and no products to sell you. All our news and educational content is free for everyone to read, but it does mean that we rely on the help of people like you. Every contribution, no matter if it’s big or small, supports independent journalism and sustains our future.