Scientists have concluded that rapamycin treatment started in early mid-life can prevent age-related blood flow impairment in the hindlimbs of wild-type, atherosclerotic, and Alzheimer’s model mice [1].
PAD and aging
Peripheral artery disease (PAD) is defined as reduced blood flow to the lower limbs. Age is a major risk factor for PAD, as the disease affects as many as 25% of people over the age of 55 and 40% of people over the age of 80. PAD is also associated with atherosclerosis, diabetes, hypertension, smoking, and Alzheimer’s disease. PAD can develop into a serious incapacitating condition and even require amputation.
Rapamycin, which is well-known for its effects on aging, has been previously demonstrated to improve cerebral blood flow in murine Alzheimer’s models and peripheral blood flow in murine models of atherosclerosis [2] by inhibiting mTORC1, a protein complex that drives growth but also promotes aging processes. This research group has previously shown that mTORC1 drives age-related cerebrovascular degeneration, decreasing cerebral blood flow in old rats [3].
Let it flow
This study began with an examination of aged but otherwise healthy wild-type mice. As expected, blood flow was significantly impaired in this group. In this initial experiment, rapamycin supplementation, started at 7 months of age, was found to completely block this age-related effect.
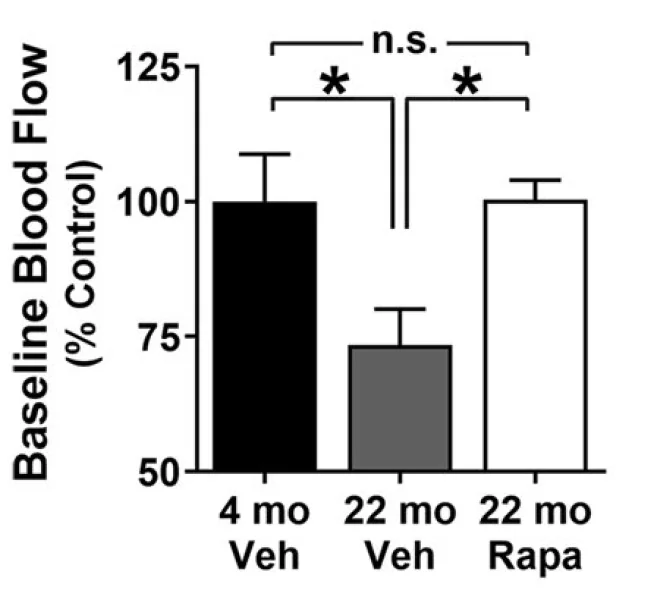
In the second experiment, the researchers used a mouse model of atherosclerosis with impaired production of LDL receptors (LDLR). To induce atherosclerosis, these mice were also fed a high-fat diet. Aged atherosclerotic animals had severely impaired blood flow, showing the converging deleterious effects of aging and the disease. Again, rapamycin treatment was able to significantly protect blood flow, although this time, it was not quite to the level of young, wild-type controls. While the difference between rapamycin-treated mice and controls was not statistically significant, it was nonetheless noticeable.

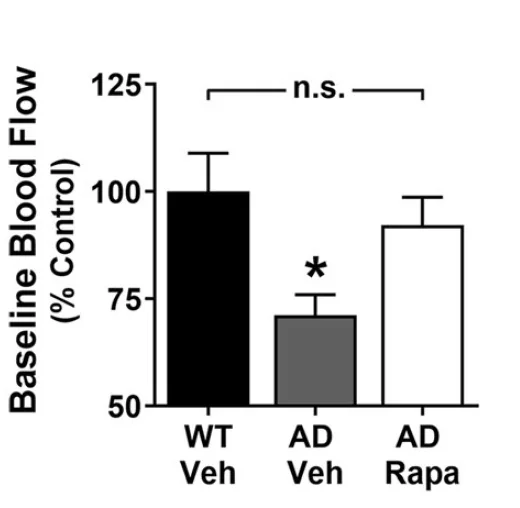
Finally, in hAPP (amyloid precursor protein) mice, an established murine model of Alzheimer’s disease, the researchers observed a similar pattern: blood flow in aged hAPP mice was severely restricted, but hAPP mice fed with rapamycin were largely protected. Here, too, the treatment with rapamycin did not block the decline in blood flow completely, but the difference between the study group and the wild-type controls did not rise to the level of statistical significance.
In all three cases, the researchers also attempted to increase blood flow by applying menthol-based ointment similar to over-the-counter products. In naturally aged mice, rapamycin treatment worked perfectly, completely preventing the age-related decline in the sensitivity of the vasculature to this treatment. However, in both atherosclerotic and hAPP mice, rapamycin only had partial effects:
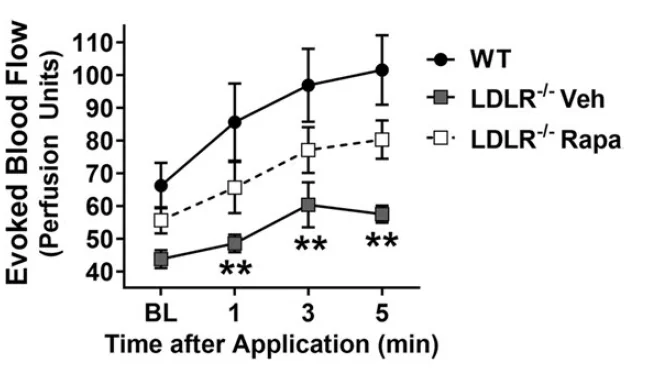
This might be explained by the converging effects of each of the diseases (AD and atherosclerosis) and aging on blood flow, with rapamycin only being able to counter the age-related portion.
Yes to NO
Menthol increases blood flow, in part, by stimulating the production of NO (nitric oxide), a potent blood flow modulator. According to previous research, mTORC1 represses NOS (nitric oxide synthase) activation, while rapamycin does the exact opposite [4]. It is worth noting, however, that a recent study produced opposite results [5]. According to the researchers, “rapamycin may improve all NO bioavailability, thus negating one of the central impairments in endothelial cell dysfunction, leading to restored vascular function in both brain and the periphery.”
The researchers note that in addition to rapamycin, other mTORC1-modulating interventions such as dietary restriction and intermittent fasting might improve peripheral blood flow and prevent peripheral artery disease, although this requires additional research.
In conclusion, our studies suggest that, in addition to central vascular impairment in aging and age-associated neurological diseases such as AD and vascular dementia, peripheral vascular decline is mediated by mTOR. Interventions that reduce mTOR activity in the vasculature thus have significant promise to prevent or treat PAD.
Conclusion
This mouse study adds to our understanding of rapamycin’s protective effects in an aging organism. PAD is a serious age-related condition that can contribute to other manifestations of aging by restricting the ability to exercise and socialize. It remains to be seen if rapamycin or other rapamycin-mimicking interventions can be effective when started later in life.
Literature
[1] Van Skike, C. E., DeRosa, N., Galvan, V., & Hussong, S. A. (2023). Rapamycin restores peripheral blood flow in aged mice and in mouse models of atherosclerosis and Alzheimer’s disease. GeroScience, 1-10.
[2] Jahrling, J. B., Lin, A. L., DeRosa, N., Hussong, S. A., Van Skike, C. E., Girotti, M., … & Galvan, V. (2018). mTOR drives cerebral blood flow and memory deficits in LDLR−/− mice modeling atherosclerosis and vascular cognitive impairment. Journal of Cerebral Blood Flow & Metabolism, 38(1), 58-74.
[3] Van Skike, C. E., Lin, A. L., Roberts Burbank, R., Halloran, J. J., Hernandez, S. F., Cuvillier, J., … & Galvan, V. (2020). mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell, 19(1), e13057.
[4] Lin, A. L., Zheng, W., Halloran, J. J., Burbank, R. R., Hussong, S. A., Hart, M. J., … & Galvan, V. (2013). Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. Journal of Cerebral Blood Flow & Metabolism, 33(9), 1412-1421.
[5] Wang, Y., Li, Q., Zhang, Z., Peng, K., Zhang, D. M., Yang, Q., … & Sun, C. (2022). mTOR contributes to endothelium-dependent vasorelaxation by promoting eNOS expression and preventing eNOS uncoupling. Communications biology, 5(1), 726.






