Scientists have successfully reversed persistent lung fibrosis in mice by overexpressing the SIRT3 protein in lung macrophages [1].
Fibrosis: not knowing when to stop
Fibrosis may be the true apex predator of humanity. According to one study, fibrotic diseases that cause organ failure are responsible for around 45% of all deaths in the United States [2]. Aging is a major risk factor for fibrosis.
Like many harmful biological processes, fibrosis is a beneficial process under normal circumstances: it is a repair mechanism that is triggered by injuries. While we are young, a balance exists between fibrosis and the subsequent regeneration of tissues. With age, our regenerative abilities falter, and fibrosis goes on unchecked.
One fibrotic disease has recently made headlines in the context of the SARS-CoV2 pandemic: idiopathic pulmonary fibrosis (IPF). Severe COVID-19 and IPF share major risk factors, including age, along with some symptoms. A study in Lancet suggests that these two diseases may also share treatments [3].
An inhaled delivery mechanism
This time, a group of researchers identified a potential target for IPF therapy that may sound familiar: SIRT3, a member of the sirtuin family of proteins. Sirtuins are highly evolutionary conserved proteins known for their effects on aging. Most of them reside in the nucleus, where they perform various maintenance tasks, such as histone deacetylation required for DNA stability. However, three members of the family are located in mitochondria. One of them, SIRT3, is a mitochondrial deacetylase, and its overexpression has been associated with increased longevity in humans [4].
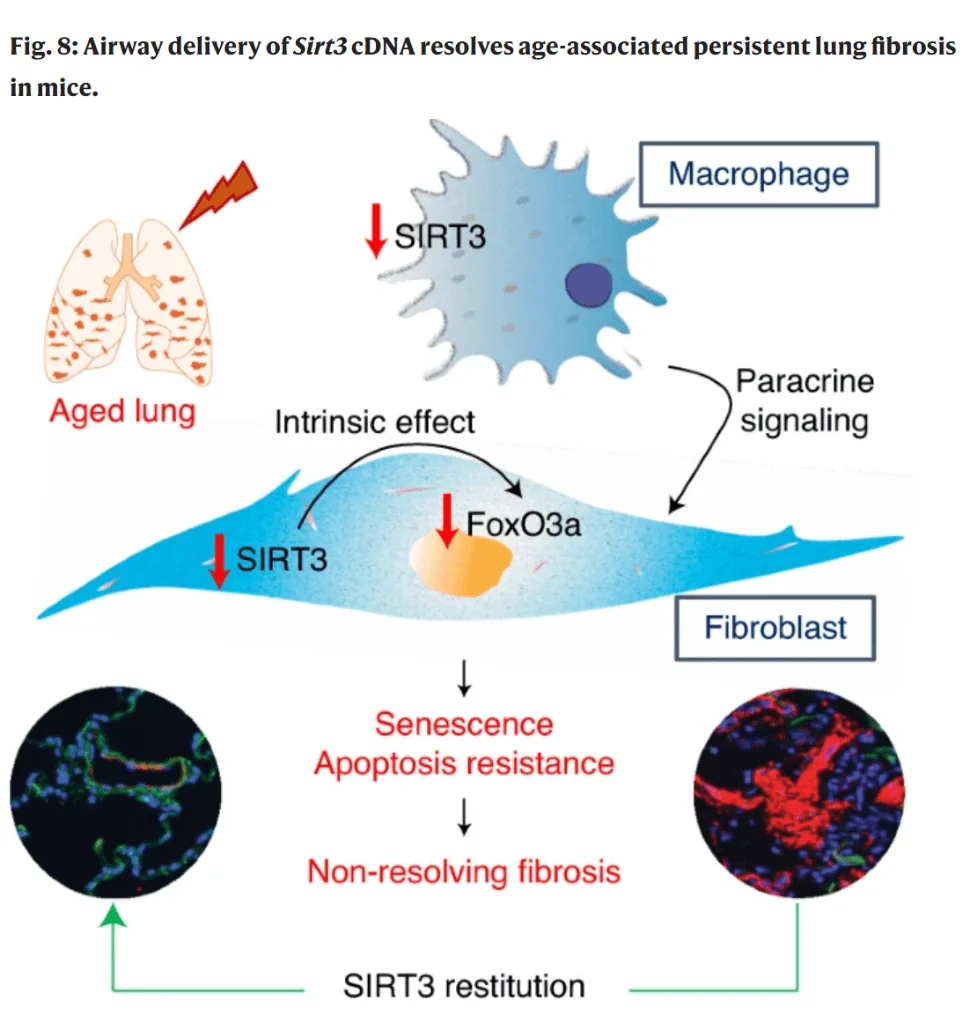
The researchers observed a considerable reduction of SIRT3 expression in the lungs of IPF patients, especially in patients with rapid progression of the disease. Findings were similar in mice with IPF-like lung injury. Consistent with the normal behavior of fibrosis, young mice gradually recovered their regenerative potential and SIRT3 levels, while mice with genetically induced accelerated aging did not. Most importantly, the scientists were able to reverse persistent lung fibrosis in aged mice by overexpressing SIRT3 via airway delivery, for which they used SIRT3-coding plasmids (small, circular DNA fragments).
Myofibroblasts and macrophages
To understand the mechanism behind this effect, the researchers started with myofibroblasts, which differentiate from resident fibroblasts. These are key effector cells that respond to lung injury and orchestrate repair. It appears that downregulation of SIRT3 is a prerequisite for myofibroblast differentiation.
Fibrosis resolution is associated with myofibroblasts undergoing apoptosis. To put it simply, after having finished their job, myofibroblasts have to die. SIRT3 deficiency in aged organisms makes these cells become resistant to apoptosis and opt for senescence instead. SIRT3 overexpression appears to alleviate this problem, while SIRT3 silencing exacerbates it. The researchers were able to show that SIRT3 achieves it indirectly via another protein, FOXO3A, a transcription factor that is associated with longevity. Interestingly, simple overexpression of SIRT3 in fibroblasts in vitro could not recapitulate all the effects of delivering SIRT3 via airway – particularly the FOXO3A upregulation. This led the researchers to hypothesize that these effects were achieved at least in part via intercellular communication. After running several cell type models, the scientists found that the effect is best explained by bringing macrophages into the equation.
In relation to fibrosis, macrophages have two strikingly different phenotypes: they can be either pro-fibrotic or anti-fibrotic [5]. In this study, macrophages seemed to act as the primary receivers of the SIRT3 plasmids. The researchers suggest that SIRT3 regulates changes in the fibrotic phenotype of macrophages. According to this hypothesis, SIRT3 downregulation in macrophages makes them pro-fibrotic. Pro-fibrotic macrophages then induce myofibroblast differentiation via cell-to-cell communication. In younger organisms, this process, when no longer needed, resolves itself by myofibroblasts undergoing apoptosis. This requires upregulation of SIRT3, but the age-related SIRT3 deficiency stands in the way. When SIRT3 is artificially supplemented in macrophages, the balance is restored: they acquire the anti-fibrotic phenotype and drive myofibroblasts towards apoptosis. It is possible that this effect is complemented by the direct uptake of SIRT3 by myofibroblasts themselves.
Conclusion
While the mechanisms by which SIRT3 restitution leads to the results demonstrated in this study need to be investigated further, there is no doubt that this research has contributed a great deal to our understanding of fibrosis and of ways to alleviate it. Their findings also confirm the importance of SIRT3, cellular senescence, and apoptosis for various aging processes. Any new insights into lung fibrosis are also highly relevant in the context of COVID-19.
Literature
[1] Rehan, M., Kurundkar, D., Kurundkar, A. R., Logsdon, N. J., Smith, S. R., Chanda, D., … & Thannickal, V. J. (2021). Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nature Aging, 1-13.
[2] Zhang, F., Ayaub, E. A., Wang, B., Puchulu‐Campanella, E., Li, Y. H., Hettiarachchi, S. U., … & Low, P. S. (2020). Reprogramming of profibrotic macrophages for treatment of bleomycin‐induced pulmonary fibrosis. EMBO molecular medicine, 12(8), e12034.
[3] George, P. M., Wells, A. U., & Jenkins, R. G. (2020). Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. The Lancet Respiratory Medicine.
[4] Bellizzi, D., Rose, G., Cavalcante, P., Covello, G., Dato, S., De Rango, F., … & De Benedictis, G. (2005). A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics, 85(2), 258-263.
[5] Zhang, F., Ayaub, E. A., Wang, B., Puchulu‐Campanella, E., Li, Y. H., Hettiarachchi, S. U., … & Low, P. S. (2020). Reprogramming of profibrotic macrophages for treatment of bleomycin‐induced pulmonary fibrosis. EMBO molecular medicine, 12(8), e12034.