Loose Chromatin, Senescent Inflammation, and Cancer
- This sheds light on how the SASP may be related to tumors.
- Senescent cells remodel their chromatin in a way that allows SASP-related compounds to be expressed more easily.
- KDM4 demethylates this chromatin, making it more accessible.
- Affecting KDM4 also appears to have benefits against cancer.
In Aging Cell, researchers have reported that chromatin demethylation allows SASP compounds to be more easily expressed.
Chromatin and gene expression

Read More

Read More
The authors of this study begin by discussing two concepts that appear only loosely related: senescence and gene expression. Both of these concepts are very well-known in aging research, but putting them directly together is not frequently seen.
However, this work does not focus on epigenetic clocks nor on epigenetic alterations more generally. Instead, these researchers are specifically focused on chromatin, the tightly wound DNA whose configuration determines what is, and isn’t, accessible for transcription. They note that a decrease in histone methylation, which makes this DNA more accessible, is associated with premature aging [1].
Specifically, the researchers focused on lysine methylation, a common form of histone methylation that is reduced by the KDM4 subfamily of demethylases. KDM4 has been heavily implicated in cancer, and KDM4 inhibitors, alongside other epigenetic-related drugs, have been investigated in the treatment of breast cancer [2]. Little previous work, however, has been done to investigate its role in cellular senescence.
Loose chromatin allows the SASP to be produced
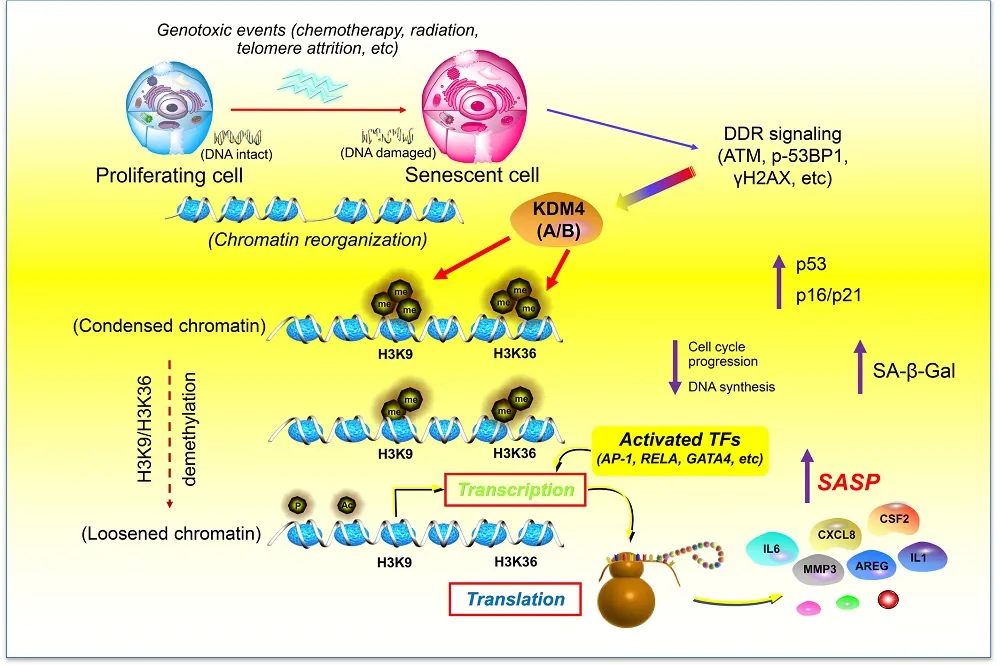
These researchers used bleomycin to induce senescence in human prostate stromal cells, which consist largely of fibroblasts, and compared their protein profiles to those of non-senescent cells. Of the 447 proteins they were able to quantify, they found that 87 were more abundant in senescent cells and 29 were less abundant. Most notably, they found that this protein expression was related to methylation; key histone signals, including H3K9 and H3K36 methylation markers, were diminished.
Further work found that this relationship between histone methylation and cellular senescence was strong. SASP expression was directly related to decreased methylation along with an increase in KDM4 and an increase in the DNA damage marker γH2AX. The researchers found that these specific changes were only relevant to cellular senescence and did not appear to apply to cancers.
Two variants, KDM4A and KDM4B, were found to drive SASP expression rather than senescence itself. Silencing both of them through short hairpin RNA (shRNA) significantly decreased core SASP factors but did not affect whether or not cells became senescent. Similarly, using other compounds to maintain histone methylation did not affect the progression of senescence; it only affected the production of inflammatory SASP factors. Furthermore, these two KDM variants were not solely responsible for the SASP; blocking NF-κB, the SASP’s master regulator, attenuated the SASP even while KDM4A and B were being promoted.
Of course, KDM4A and B, having such broad effects on methylation, do not affect only compounds related to senescence. Suppressing their effects through the compound ML324 led to the downregulation of many proteins, not just those relating to the SASP. The researchers recommend further work on determining what exactly happens to cells that are suppressed in this way. The researchers did confirm, however, that senescent cells differ from normal cells in which genes are more accessible through chromatin, which appears to explain why histone methylation has such strong effects on the SASP.
Relationship to cancer
Much of this work was focused on cancer rather than senescence. Crucially, the researchers found that upregulated KDM4A and B, and the decreased methylation that they represent, are correlated with a reduced chance of disease-free survival. These compounds did not appear to have relevant effects on the cancer cells themselves but rather the cancer stroma: the noncancerous portions of a tumor that support its growth.
Utilizing a mouse model with diabetes and immunodeficiency, the researchers investigated ML324 and the genotoxin MIT as a potential treatment for cancer. Two weeks after being injected with cancer, the treatment groups of mice were given one or both of these compounds for eight weeks.
Mirroring the cellular study, growths that originated from pure cancer cells were not affected by ML324. However, when these cancer cells were accompanied by stromal cells, administering ML324 alongside MIT substantially decreased the size of the tumors compared to MIT alone. This lends weight to the idea that affecting KDM4, or perhaps the SASP itself, may be effective in prolonging survival and helping people fight cancer when used alongside more common tools. However, much more work will need to be done to determine if this approach can be used to fight prostate or any other type of cancer.
Of note is that this work bears the name of the late Judith Campisi. This is because it was originally published in Nature Aging in 2021 but was later retracted due to concerns regarding its images. This Aging Cell article was submitted after addressing these issues.
Literature
[1] Gruenbaum, Y., & Foisner, R. (2015). Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annual review of biochemistry, 84(1), 131-164.
[2] Metzger, E., Stepputtis, S. S., Strietz, J., Preca, B. T., Urban, S., Willmann, D., … & Schüle, R. (2017). KDM4 inhibition targets breast cancer stem–like cells. Cancer research, 77(21), 5900-5912.







