An international collective of scientists has discovered a new cellular process: telomere transfer from antigen-presenting cells to T cells that boosts the latter’s lifespan and proliferative potential [1].
Read More
T cells, an important element of our adaptive immune system, are born as non-proliferating cells and, as such, do not experience telomere attrition. However, they do begin to actively proliferate upon activation by antigens, which is when telomere attrition starts taking its toll. Abnormally short telomeres are found in T cells from people who are infected with certain viral pathogens, such as HIV, or in chronically stimulated T cells from patients with inflammatory diseases. Telomere shortening in T cells is a major factor in immunosenescence.
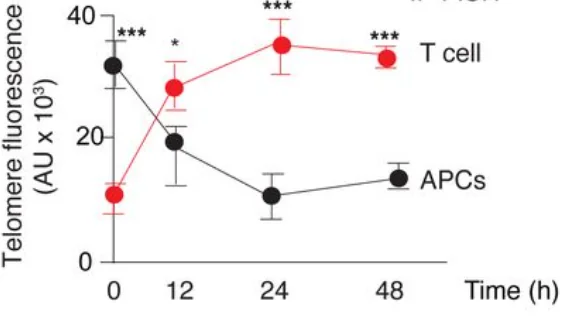
The authors of this paper came across an intriguing phenomenon: as T cells contacted antigen-presenting cells (APCs), the average length of telomeres in the T cells increased by about 3000 base pairs. Simultaneously, the average length of telomers in the APCs decreased by the same amount. The dynamic of telomere growth/attrition over time showed a perfect reverse correlation.
After a series of experiments, the scientists found that the effect only existed when APCs were “loaded” with antigens and when T cells established physical contact with APCs via so-called immunological synapses.
It has been known that T cells are able to restore their telomeres by upregulating TERT (telomerase reverse transcriptase), though this mechanism works less and less well with each activation of a T cell [2]. Nevertheless, the researchers explored the opposite hypothesis: that contact with APCs somehow boosted TERT production in T cells instead of dampening it. Unsurprisingly, this was not the case, as T cells with the TERT gene knocked out still experienced telomere lengthening upon interactions with APCs.
Another mechanism of telomere lengthening is called ALT (alternative lengthening of telomeres) [3]. It is used by a broad range of cancer cells for unchecked replication, but this mechanism requires DNA synthesis. After the researchers inhibited all DNA synthesis in the T cells, they still demonstrated lengthening of telomeres, so ALT had to be ruled out as well.
It became evident that the telomere lengthening in T cells can only be caused by their synaptic interaction with APCs. The researchers detected telomere clustering (basically, congregation of telomeres in one place) in about 70% of the immunological synapses when antigens were present but only in 10% when they were not. When APCs were not coupled with T cells, no telomere clustering occurred at all. Therefore, APCs appear to cluster their telomeres at the synapse prior to telomere transfer.
Telomere DNA was then discovered outside the APCs. Since it could not be destroyed by a DNA-cleaving compound, the researchers suggested that these DNA fragments were enclosed in protective lipid vesicles, which they proved experimentally. Another compound found in the same vesicles was TZAP, the telomere-trimming factor that is apparently involved in the cleavage of the telomere fragments from their chromosomes inside APCs. The researchers found that silencing TZAP derailed the whole telomere transfer process.
By chemically labelling APC telomeres, the researchers proved that the telomere additions detected in the T cells indeed originated in the APCs. They were also able to isolate telomere-transporting vesicles. Transferred directly into T cells, these vesicles caused telomere lengthening, providing the final proof.
Conclusion
This discovery of a previously unknown cellular process has therapeutic potential. The researchers claim that a single APC-originated telomere transfer can rescue ultra-short telomeres in near-senescent T cells and boost their proliferation more effectively than TERT, which only adds 100-200 base pairs per activation. They also hypothesized that “a loss of telomere transfer capacity rather than a loss of telomerase is responsible for telomere shortening at the basis of ageing”. It may be possible to replicate and use this effect against various immunosenescence-related conditions.
Literature
[1] Vaz, B., Vuotto, C., Valvo, S., D’Ambra, C., Esposito, F. M., Chiurchiù, V., … & Lanna, A. (2020). Intercellular telomere transfer extends T cell lifespan. bioRxiv.
[2] Barsov, E. V. (2011). Telomerase and primary T cells: biology and immortalization for adoptive immunotherapy. Immunotherapy, 3(3), 407-421.
[3] Cesare, A. J., & Reddel, R. R. (2010). Alternative lengthening of telomeres: models, mechanisms and implications. Nature reviews genetics, 11(5), 319-330.