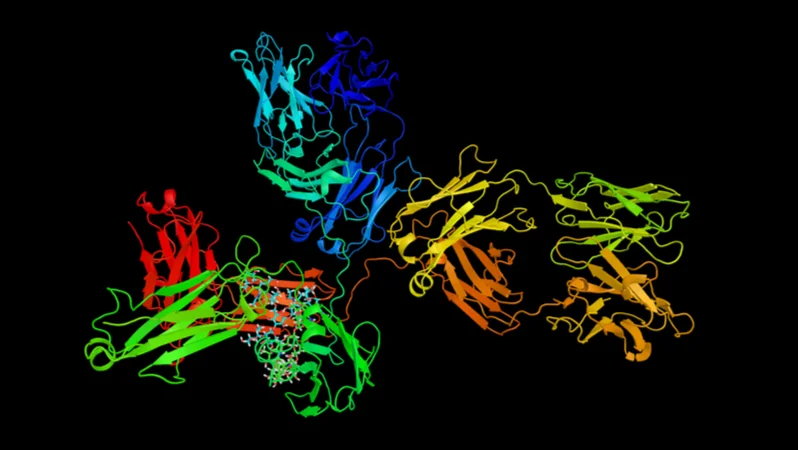
In a new review published in Clinica Chimica Acta, researchers from the University of Zagreb discuss immunoglobulin G glycans, the changes that their composition undergoes with aging, and their potential as biomarkers of aging [1].
Glycans are not AGEs
One of the review’s co-authors is Prof. Gordan Lauc, who gave a presentation on the importance of glycans in aging at Ending Age-Related Diseases 2022. He is a professor at the University of Zagreb, Director of the National Centre of Scientific Excellence in Personalised Healthcare, a co-director of the Human Glycome project, the founder of the biotech company Genos, and the CSO of GlycanAge.
Glycans are glycosylated proteins: proteins with added sugars to specific amino acids. While glycation and glycosylation are post-translational protein modification processes performed by carbohydrates, they are critically different. Glycation is non-enzymatic (random) chemical damage to a protein, and it is often discussed in pathology and as a hallmark of aging. This reaction often results in the formation of advanced glycation end-products (AGEs). Glycosylation is an enzymatic (controlled) reaction, which enriches the structure and function of a protein by turning it into a glycan.
During his EARD2022 talk, Gordan admitted that studying proteins without taking into account glycans is like studying birds without feathers: you can get a lot of insights, but you will never see the bird in flight. He emphasizes that the alternative glycosylation that proteins undergo, which places different glycan structures on the same glycosylation site, is functionally similar to genetic mutations; therefore, its study is extremely important in understanding the proteins’ functions. However, not many people study glycans due to the profound technical expertise required, particularly in mass spectrometry.
IgG glycans
The review focuses on Immunoglobulin G (IgG) glycans, which are structurally and functionally different post-translationally modified antibodies that modulate inflammation. Here, the term “glycan” is used to mean both a glycosylated protein (in this case, an IgG antibody) and a complex carbohydrate attached to the protein. IgG without glycans is almost non-functional, while an attached glycan determines an IgG protein’s function.
Autoimmune diseases, hereditary diseases, cancer, age-related diseases, and aging itself are all accompanied by a change in the IgG glycosylation pattern, which is often associated with disease severity. Understanding changes in IgG glycosylation patterns could therefore give insights into the mechanisms of aging and disease.
IgG are important antibodies that participate in adaptive immunity and link innate and adaptive immune responses. They are among the most abundant proteins in human serum and the most common antibody type. In humans, IgG antibodies are subdivided into four classes, depending on which receptors/components they bind to. Just like other antibodies, IgG are produced by B cells and are composed of an antigen-binding Fab fragment, which recognizes such things as pathogens and toxins, and a receptor-binding Fc fragment, which triggers an immune response.
IgG glycans are synthesized by different enzymes and other proteins. Both Fab and Fc fragments contain glycosylation sites. Over 30 different glycans attached to IgG have been identified. All IgG glycans can then be subdivided into several groups according to their composition. The glycans attached to IgG define various properties of the antibody, such as its structural stability, aggregation, and binding characteristics.
Age-related IgG glycan composition changes
Previous studies have shown that aging is associated withchanges in IgG glycosylation profiles: certain types of IgG glycans increase, while others decrease with age. Moreover, in many studies, gender-specific differences in the rate of change was observed, with menopause being a significant modifier of female IgG glycan composition. At the same time, different populations demonstrate similar IgG glycosylation changes, indicating a common mechanism underlying these changes.
Interestingly, the changes in IgG glycosylation profiles in people with age-related or other diseases with inflammatory components correspond to the changes associated with older age. This means a person suffering from any inflammation-causing disease is biologically older than a healthy person of the same age. It is not clear if these changes are a consequence or an underlying mechanism of a disease. The answer to this question is probably different in each case.
Age-associated changes in IgG glycan composition are obviously related to the changes that the B cells producing them undergo with aging. These include a reduced production of B cells, an accumulation of atypical B cells, and a reduction of naive B cells. Interestingly, an analysis of glycosylation profiles at different ages of another class of antibodies, IgA, did not show any differences. This might indicate IgG-specific age-related changes, although other classes are simply less abundant and are thus more difficult to analyze.
The mechanism underlying age-associated changes in IgG glycan composition is not clear. Several hypotheses were suggested, including changes in the expression of certain enzymes, which add or remove sugars from IgG glycans, and changes in the turnover of different IgG glycans. However, experimental evidence is conflicting; thus, none of the hypotheses currently prevails.
Although the underlying mechanism is not clear, it seems that the IgG glycosylation profile becomes more pro-inflammatory with age. It is known that aging is accompanied by a chronic low-level inflammation state: inflammaging. It is also acknowledged that people age at different rates. Long-lived individuals have less inflammation and younger IgG glycosylation profiles.
The authors of the review then explain that IgG glycan composition analysis can be used as a biomarker of aging. People who are in poorer health and lead an unhealthy lifestyles, such as smokers and the obese, are estimated to be older by their IgG glycan compositions. GlycanAge already offers a commercially available test to measure people’s biological ages, which is based on the analysis of 24 glycans attached to IgG. Moreover, the company is developing MenoAge, a glycomics-based blood biomarker for female aging, which was also presented at EARD2022 by Marina Kavur.
Abstract
Immunoglobulin G (IgG) antibodies are post-translationally modified by the addition of complex carbohydrate molecules – glycans, which have profound effects on the IgG function, most significantly as modulators of its inflammatory capacity. Therefore, it is not surprising that the changes in IgG glycosylation pattern are associated with various physiological states and diseases, including aging and age-related diseases. Importantly, within the inflammaging concept, IgG glycans are considered not only biomarkers but one of the molecular effectors of the aging process. The exact mechanism by which they exert their function, however, remains unknown. In this review, we list and comment on, to our knowledge, all studies that examined changes in IgG glycosylation during aging in humans. We focus on the information obtained from studies on general population, but we also cover the insights obtained from studies of long-lived individuals and people with age-related diseases. We summarize the current knowledge on how levels of different IgG glycans change with age (i.e., the extent and direction of the change with age) and discuss the potential mechanisms and possible functional roles of changes in IgG glycopattern that accompany aging.
Conclusion
Longevity researchers are constantly looking for ways to measure biological age to use as a reliable metric that can estimate both the pace of aging and the effectiveness of anti-aging interventions. IgG glycosylation-based clocks could be just what is needed, especially for certain applications such as optimizing hormone replacement therapy for women undergoing perimenopause, the stage preceding menopause, and menopause itself.
Literature
[1] Krištić, J., Lauc, G. & Pezer, M. Immunoglobulin G glycans – Biomarkers and molecular effectors of aging. Clin. Chim. Acta 535, 30–45 (2022).