Scientists have demonstrated that full-body regeneration in cnidarians, a group of animals that includes the jellyfish and hydra, can be driven by signals from senescent cells [1]. This might be the original purpose of cellular senescence.
Why can’t we regrow our arms?
There are many examples of amazing regenerative abilities in the animal kingdom, but the vast majority of complex animals, including humans, lack them. Barring a few exceptions, substantial regeneration seems to be reserved for ancient, simpler animals, such as the hydra or another cnidarian and the star of this new study, Hydractinia symbiolongicarpus.
According to the study’s authors, the reason might be that “the high plasticity that allows for whole-body regeneration may also compromise the integrity of complex structures and increase malignancy risk.” Despite the literal abyss between us and animals like hydractinia, studying regeneration in them might be relevant to human longevity if we can understand and harness some of their regeneration mechanisms.
Head to body
Hydractinia can regrow an amputated head in just three days. This regeneration is driven by adult pluripotent stem cells called i-cells. Those cells normally reside in the stems of hydractinia, ready for regenerative action. After these animals are decapitated, those cells migrate towards the injury site and facilitate head regrowth:
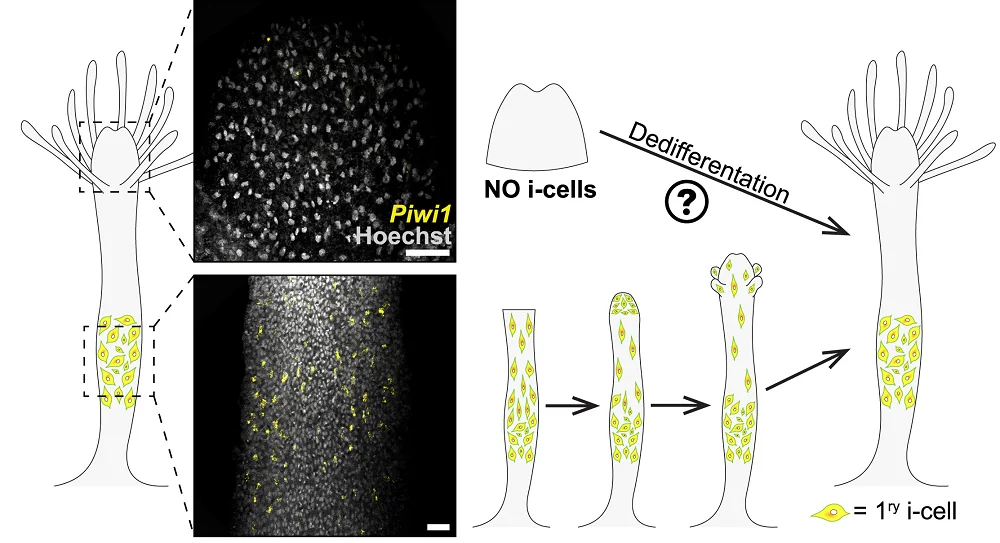
However, while hydractinia can grow new heads from their bodies, their heads lack the i-cells needed to grow new bodies with. Yet, surprisingly, amputated hydractinia heads transform into fully functional animals due to the appearance of new i-cells, which the researchers dubbed “secondary i-cells”.
Senescent cells signal regeneration
The researchers were able to establish that those cells had dedifferentiated from somatic cells. Since cellular senescence has been linked to regeneration by earlier studies, the researchers analyzed the transcription levels of senescence-related genes several times during the process of regeneration (which typically took about six days).
142 of 229 homologs of human senescence genes were differentially expressed at least once. The signal was strongest on day one, shortly after amputation, and then gradually subsided. This senescence-related activity seemed to emerge at the injury site before propagating to other parts of the amputated head.

Read More
Given that senescent cells don’t just disappear but tend to linger in tissue [2], the researchers were puzzled by the fizzling out of senescence markers. They were able to show that those senescent cells migrate to the gastrointestinal tissue of the newly grown body and then are expelled, probably through the mouth. Unfortunately, humans cannot just cough their senescent cells out.
To further investigate the role of senescent cells in the regeneration event, the researchers treated the amputated heads with navitoclax, an effective senolytic. As navitoclax inhibited senescent markers, no secondary i-cells appeared, and no regeneration event occurred. Animals with genetically ablated cellular senescence were able to regrow a head but not a body, since they had primary i-cells but no secondary i-cells.
Finally, the researchers developed a conditional model in which cellular senescence could only be induced by exposing animals to blue light. Following decapitation, only the animals that were exposed to light demonstrated cellular senescence at the injury area, then emergence of secondary i-cells, and, finally, full body regeneration.
Conserved mechanism
Those results led the researchers to believe that senescent cells emerged in amputated heads to initiate a cascade of signals that triggered dedifferentiation of somatic cells into secondary i-cells. This points to cellular senescence as a highly conserved regeneration-related mechanism that was stripped of its impressive powers in most of the more complex animals. It was repurposed (as evolution has done many times) to facilitate regeneration on a smaller scale: in wound healing [3]. Interestingly, proximity to senescent cells is known to boost cellular reprogramming with Yamanaka factors [4].
This study does not immediately show a way for humans to regrow limbs, but it expands our understanding of the fascinating phenomenon of cellular senescence. While it does play a role in aging, there is a bright side to senescence, which we might eventually learn to use to our advantage.
We suggest that senescence is an ancient mechanism, instructing cells adjacent to an injury site to prepare for a regenerative event. We also speculate that other consequences of senescence that have been observed in mammals, such as long-term retention and accumulation of senescent cells, aging, chronic inflammation, and cancer, are side effects that evolved later in the evolution of these lineages, perhaps as a consequence of the increase in cell fate stability and morphological complexity. Understanding the senescent environment and its role in cellular plasticity could pave the way for new treatments to enhance regeneration in poorly regenerating mammals.
Literature
[1] Salinas-Saavedra, M., Krasovec, G., Horkan, H. R., Baxevanis, A. D., & Frank, U. (2022). Senescence-induced cellular reprogramming drives cnidarian whole-body regeneration. Cell Reports.
[2] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[3] Adams, P. D. (2009). Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Molecular cell, 36(1), 2-14.
[4] Mosteiro, L., Pantoja, C., Alcazar, N., Marión, R. M., Chondronasiou, D., Rovira, M., … & Serrano, M. (2016). Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science, 354(6315), aaf4445.




