Dr. Belmonte’s group at Altos Labs targeted stressed and senescent cells with partial reprogramming, producing large increases in lifespan in male mice [1].
What are they doing there?
Since the discovery of cellular reprogramming almost two decades ago, a lot of hopes have been put into this technology, and a lot of progress has been made. Partial reprogramming, when cells do not revert to a pluripotent state but retain their identity while also undergoing rejuvenation, is being pursued by numerous companies, including the gigantic and secretive Altos Labs, which was founded by Jeff Bezos and Yuri Milner with a three-billion-dollar investment.
Altos Labs has recruited many big names in the longevity field, such as Steve Horvath, Morgan Levin, and Juan Carlos Izpisua Belmonte. Dr. Belmonte was the first to demonstrate that partial cellular reprogramming extends lifespan in fast-aging (progeroid) mice [2]. For a couple of years, little has been known about what’s going on at Altos. A new paper in Science by Dr. Belmonte’s team might be a major leap towards bringing partial cellular reprogramming to the clinic.
Targeting only damaged cells
The scientists used just three of the original four reprogramming “Yamanaka factors”: OSK instead of OSKM. Omitting the fourth factor, c-Myc, was pioneered by Harvard geroscientist David Sinclair, and his team’s work on partial reprogramming of retinal ganglion cells [3] was given credit in this new paper. The three-factor cocktail is thought to be safer and easier on cells.
However, this study’s big distinction is that the viral vectors carrying the three factors were only aimed at stressed and senescent cells. While there have been experiments with tissue-specific reprogramming, the novelty in Dr. Belmonte’s approach is that it targets damaged, but not healthy cells in multiple tissues. The hypothesis was that rejuvenating those cells might be enough for a robust effect on an organismal level.
The targeting was achieved by using the promoter Cdkn2a, which is mostly active in stressed and senescent cells. If the environment in those cells turns the endogenous promoter on, it should also activate the same promoter on the viral vector, triggering OSK expression.
Late-life treatment increases lifespan
First, the researchers experimented with a mouse model of Hutchinson-Guilford progeria syndrome. Such mice experience greatly accelerated aging. The OSK treatment led to improvements in both median (40%) and maximal (32%) lifespan, body weight, activity, and inflammation.
As a positive control, the researchers used viral vectors with anti-inflammatory cargo (an NF-κB inhibitor) to make sure the effects produced by the OSK treatment go beyond simply quelling inflammation. Indeed, the anti-inflammatory treatment led to a much smaller increase in lifespan.
The improvements in lifespan were comparable to the group’s previous results with full-body cellular reprogramming, suggesting that targeting only damaged cells can be just as effective. However, in that experiment, the researchers used all four reprogramming factors.
Progeroid mice are not the best models of natural aging. The researchers subsequently moved to wild-type mice, delivering a single injection to aged (18-month-old) male animals. Despite the late-life administration, the treatment significantly improved both median and maximal lifespan (median by 12%). Age-related body weight loss was largely prevented, and overall physical activity and fitness were improved compared to age-matched controls.
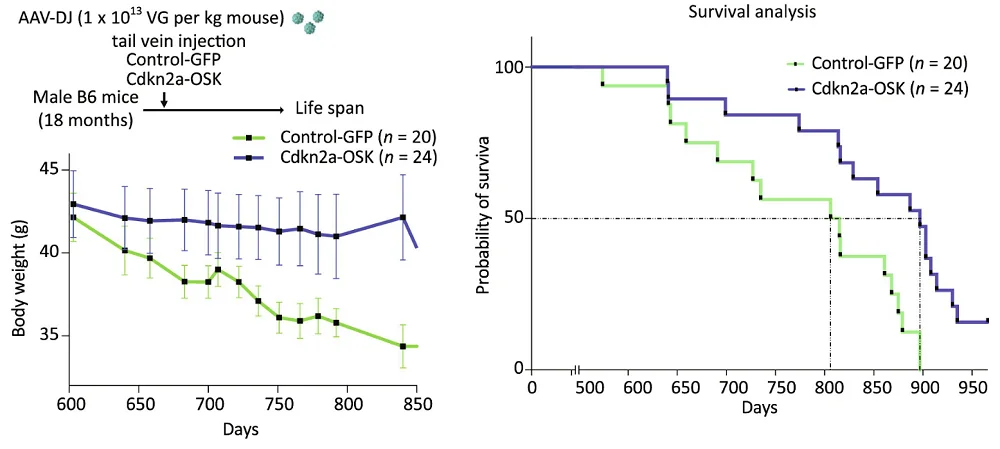
Not a senolytic
The researchers investigated whether the treatment killed senescent cells (a senolytic effect). However, eight months after the shot, most cells where viral-delivered OSK were activated were still there. This shows that the treatment improved the targeted cells instead of just eliminating them.
Senescent cells are not always harmful. In fact, they exist even in young people and play important roles in development, wound healing, and cancer prevention, although they can also promote cancer under certain circumstances. This is why a senomorphic approach, which alters senescent cells towards a healthier phenotype, might be superior. Reassuringly, the OSK treatment improved wound healing in the mice.
Another known problem with cellular reprogramming is that it might lead to the creation of tumors (tumorigenesis), although OSK without the M has a better safety profile. In this study, too, the treated mice were not more prone to cancer than controls over two years of follow-up.
The researchers note that the way their treatment downregulates pro-inflammatory genes in senescent cells without leading to cell death resembles how some other geroprotective interventions work, including metformin and the mTOR inhibitor rapamycin.
These findings suggest that we may not need to target a large population of cells to elicit functional organismal improvement. Young organisms have the potential to cope with a diverse range of stresses, with a strong molecular buffering capacity that gradually deteriorates with age. This buffering capacity may be improved by targeting a small population of cells, such as aged and stressed cells, leading to the improvement of the entire organism. Further understanding of the target organs and cell types driving the beneficial effects of Cdkn2a-OSK may allow us to develop a more precise approach to achieve organismal rejuvenation and reverse disease phenotypes.
Literature
[1] Sahu, S. K., Reddy, P., Lu, J., Shao, Y., Wang, C., Tsuji, M., … & Belmonte, J. C. I. (2024). Targeted partial reprogramming of age-associated cell states improves markers of health in mouse models of aging. Science Translational Medicine, 16(764), eadg1777.
[2] Ocampo, A., Reddy, P., Martinez-Redondo, P., Platero-Luengo, A., Hatanaka, F., Hishida, T., … & Belmonte, J. C. I. (2016). In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell, 167(7), 1719-1733.
[3] Lu, Y., Brommer, B., Tian, X., Krishnan, A., Meer, M., Wang, C., … & Sinclair, D. A. (2020). Reprogramming to recover youthful epigenetic information and restore vision. Nature, 588(7836), 124-129.




