Researchers publishing in Journal of Nanobiotechnology have found that extracellular vesicles derived from young cells are effective in treating a model of a common tendon disease.
Tendinopathy and stem cells
Swelling, weakness, tenderness, and tendon pain during activities are the characteristic symptoms of chronic tendinopathy [1], a condition that is exacerbated by aging and obesity into degeneration and rupture [2]. This condition is notoriously difficult to treat, and even surgery is poorly effective [3].
Previous research has investigated potential treatments that involve tendon stem/progenitor cells (TSPCs) and mesenchymal stem cells (MSCs) [4]. Adipose-derived MSCs (ADMSCs) have a higher yield and quality than other stem cell sources, and they have previously been used to treat tendinopathy in a rat model [5].
One of the core parts of MSC effectiveness involves extracellular vesicles (EVs), which have been explored in treating multiple diseases, including tendinopathy [6]. However, donor EVs are strongly affected by the donor’s health, including aging [7].
Read More
NAMPT, a core component of NAD+ synthesis, declines with age [8] and is contained in EVs. Hypothesizing that NAMPT is an important part of tendinopathy recovery through EV administration, the researchers designed an experiment that compares the EVs of young and old animals.
Graphical abstract
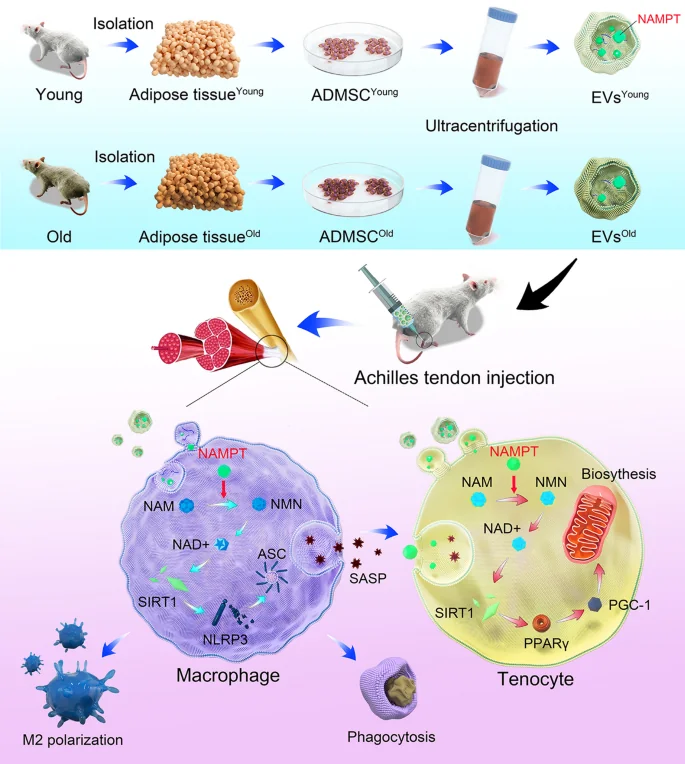
Comparing MSCs in animals and cells
For the first part of their experiment, the researchers compared MSCs from 22-month-old mice to 2-month-old mice. As expected, the older mice had significantly more senescent MSCs, as measured by the well-known senescence biomarkers p16INK4A, p21, and SA-β-gal, along with significantly decreased NAD+ and NAMPT.
This decrease in NAMPT was also found to apply to these MSCs’ EVs; EVs derived from old MSCs had less than half the NAMPT of their younger counterparts. Interestingly, old MSCs generated more than twice as many EVs as young MSCs did, sacrificing quality for quantity, which the researchers attribute to inflammatory stress.
Similar effects were also found in macrophages. Administering IL-1β to macrophages caused signs of cellular senescence, which were ameliorated by young-derived EVs. These EVs also encouraged macrophages to take up the M2 (healing) phenotype, as opposed to the M1 (inflammatory) phenotype, and they decreased inflammatory signals given off by macrophages.After inducing tendinopathy in mice through collagenase I, the EVs’ effects on the disorder were examined. EVs derived from older animals had a minimal effect on biomarkers, including matrix metalloproteinase and collagen levels. Microscopic examination revealed similar results, with tendon fibrosis ameliorated only by young-derived EVs.
In a cellular study, the resarchers simulated tendinopathy by exposing tenocytes, which are fibroblasts found only in the tendons, to IL-1β. The affected cells experienced death by apoptosis at much higher rates, but this was almost entirely ameliorated by young-derived EVs. Old-derived EVs had a negligible effect.
These results were recapitulated in an examination of gene expression. Tendinopathy causes pathological remodeling of the extracellular matrix, but this too was ameliorated by young-derived EVs. Levels of collagen-related gene expression were also restored.
The connection with NAMPT and NAD+
Finally, the researchers examined the cells’ biochemistry to determine what effects the NAMPT-rich EVs were having on tenocytes exposed to IL-1β. An NAD+ signaling pathway that was substantially downregulated by IL-1β was largely restored to previous values. The mitochondria were found to be significantly and positively affected, including such metrics as oxygen respiration, energy production, and membrane integrity. Once again, only the young-derived EVs were successful in these respects.
Conclusion
The researchers describe their approach as “one stone, two birds”, emphasizing the fact that young EVs can both directly restore tenocytes and discourage macrophages from making things worse with inflammation. If clinical trials prove that these effects occur in human beings as well as mouse and cellular models, chronic tendinopathy may become a more easily treatable condition along with other age-related diseases.
Literature
[1] Millar, N. L., Silbernagel, K. G., Thorborg, K., Kirwan, P. D., Galatz, L. M., Abrams, G. D., … & Rodeo, S. A. (2021). Tendinopathy. Nature reviews Disease primers, 7(1), 1.
[2] Gomez-Florit, M., Labrador-Rached, C. J., Domingues, R. M., & Gomes, M. E. (2022). The tendon microenvironment: engineered in vitro models to study cellular crosstalk. Advanced Drug Delivery Reviews, 114299.
[3] Dan, M., Phillips, A., Johnston, R. V., & Harris, I. A. (2019). Surgery for patellar tendinopathy (jumper’s knee). Cochrane Database of Systematic Reviews, (9).
[4] Schneider, M., Angele, P., Järvinen, T. A., & Docheva, D. (2018). Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Advanced drug delivery reviews, 129, 352-375.
[5] Oshita, T., Tobita, M., Tajima, S., & Mizuno, H. (2016). Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. The American Journal of Sports Medicine, 44(8), 1983-1989.
[6] Chen, S. H., Chen, Z. Y., Lin, Y. H., Chen, S. H., Chou, P. Y., Kao, H. K., & Lin, F. H. (2021). Extracellular vesicles of adipose-derived stem cells promote the healing of traumatized Achilles tendons. International Journal of Molecular Sciences, 22(22), 12373.
[7] Hooten, N. N., Byappanahalli, A. M., Vannoy, M., Omoniyi, V., & Evans, M. K. (2022). Influences of age, race, and sex on extracellular vesicle characteristics. Theranostics, 12(9), 4459.
[8] Yoshida, M., Satoh, A., Lin, J. B., Mills, K. F., Sasaki, Y. O., Rensing, N., … & Imai, S. I. (2019). Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell metabolism, 30(2), 329-342.