There has been a lot of hope and hype around blood transfusions and it being able to reverse aging recently. We decided to take a look at the science behind the idea and talk with a leading expert in the field to see what the reality was.
Is the blood a key to aging?
Due to a recently published study on the effects of young plasma on aged mice, we got in touch with Dr. Irina Conboy of the University of California Berkeley. Dr. Conboy is an Associate Professor at the Department of Bioengineering and an expert in stem cell niche engineering, tissue repair, stem cell aging and rejuvenation. Before we dive into the main topic, let’s familiarize ourselves a little with Dr. Conboy and her work.
Dr. Conboy got her Ph.D. at Stanford University, focusing on autoimmunity. She met her partner in science—and in life—Dr. Michael Conboy at Harvard and they got married before embarking on graduate studies; they celebrated their Silver Anniversary a few years ago. During her postdoctoral studies, she began focusing on muscle stem cells, trying to figure out what directs them to make new healthy tissue and what causes them to lose their ability to regenerate the tissues they reside in as we age [1].
Together with her husband Michael, she eventually discovered that old stem cells could be reactivated and made to behave like young ones if appropriately stimulated. The Conboys’ parabiosis experiments—which consisted in hooking up the circulatory systems of aged and young mice—showed that old age is not set in stone and can be reversed in a matter of weeks [2].
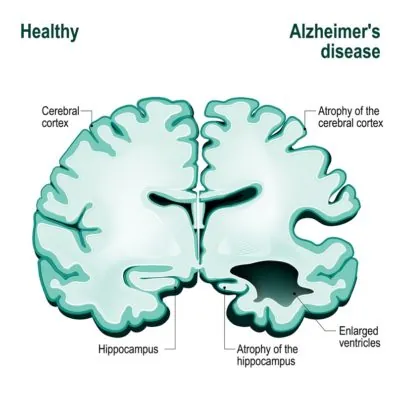
The follow-up work by the Conboys uncovered that age-accumulated proteins, such as TGF-β1, inhibited stem cells’ ability to repair tissues even in young mice, and when TGF-β1 signaling is normalized to its young levels, old mice (equivalent to 80-year old people) have youthful muscle regeneration and better neurogenesis in the hippocampus (the area of the brain that is responsible for memory and learning)[3].
While young blood did appear to be beneficial to old stem cells, their evidence suggested that the real culprit of the broad loss of tissue repair with age was the negative influence of age-accumulated inhibitory proteins in aged tissues and circulation, also called the stem cell niche [4].
The results support that aging is at least partly due to damage accumulation
This conclusion is certainly compatible with the view of aging as a damage accumulation process [5]. As Irina herself pointed out in this interview, in the parabiosis experiments, the old mice had access to the more efficient young organs: lungs, liver, kidneys and immune system of the younger mice, which likely accounted for many of the benefits observed in the elderly parabiosed mice. With respect to the rejuvenation of the brain, the old mice experienced environmental enrichment by being sutured to young, more active parabionts, and this is known to improve the formation of new brain cells, learning, and memory.
An aged niche blocks the action of old and young stem cells alike very quickly; therefore, as Dr. Conboy observed in an article in the Journal of Cell Biology, we can’t treat the diseases of aging by simply transplanting more stem cells, because they will just stop working. Their niche needs to be appropriately engineered as well. Fortunately, there are potential solutions to this problem; such as the use of artificial gel niches and defined pharmacology that are designed to protect transplanted or endogenous stem cells from the deleterious environment of the old body.
This research holds the potential to significantly postpone the onset of age-related diseases and possibly reverse them one day, including frailty, muscle wasting, cognitive decline, liver adiposity and metabolic failure, but Dr. Conboy remains cautious about the possibilities until more data is in. However, she does think that longer and healthier productive lives could improve people’s attitudes towards the environment and treating each other with compassion and respect—a view that we definitely share.
We managed to catch up with Irina and Michael Conboy and talk to them about their work.
For the sake of those new to the topic, what is it in young blood and aged blood that affects aging?
Irina: Numerous changes in the levels of proteins that together regulate cell and tissue metabolism throughout the body.
Mike: We wondered why almost every tissue and organ in the body age together and at a similar rate, and from the parabiosis and blood exchange work now think that young blood has several positive factors, and old blood accumulates several negative, “pro-aging” factors.
A lot of media attention and funding is currently being directed to youthful blood transfusions; how can we move beyond this to potentially more promising approaches, such as filtering and calibration of aged blood?
Irina: People need to understand not just the titles, abstracts and popular highlights of research papers, but the results and whether they support (or not) the promise of rejuvenation by young blood. In contrast to vampire stories, we have no strong experimental evidence that this is true, and there is a lot of evidence that infusing your body with someone else’s blood has severe side effects (even if it is cell-free).
Mike: Translational research!
Some evidence suggests dilution is the most likely reason that young blood has some beneficial effects; what are your thoughts on this recent study[6] in rats that shows improved hepatic function partially via the restoration of autophagy?
Irina: There are certainly “young” blood factors that are beneficial, not just a dilution of the old blood, and this benefit differs from organ to organ. We have published on improved liver regeneration, reduced fibrosis and adiposity by transfusion of old mice with young blood, but these are genetically matched animals, and in people, we do not have our own identical but much younger twins[7].
If dilution is also playing a role here, then can we expect similar or better results from calibrating aged blood?
Irina: Yes, and our work in progress supports the idea.
In your 2015 paper, you identified that TGF-β1 can be either pro-youthful or pro-aging in nature, depending on its level[8]. In the study, you periodically used an Alk-5 inhibitor to reduce TGF-β1 levels and promote regeneration in various tissues. In the study, you showed that TGF-β1 was important in myogenesis and neurogenesis; is there reason to believe that this mechanism might be ubiquitous in all tissues?
Irina: Yes, because TGF-β1 receptors are present in most cells and tissues.
Also, TGF-β1 is only one of a number of factors that need to be carefully balanced in order to create a pro-youthful signalling environment. How many factors do you believe we will need to calibrate?
Irina: There will be a certain benefit from calibrating just TGF-beta 1, but also additional benefits from more than one or just TGF-beta.
How do you propose to balance this cocktail of factors in aged blood to promote a youthful tissue environment?
Irina: We are working on the NextGen blood apheresis devices to accomplish this.
So, you are adapting the plasmapheresis process to effectively “scrub” aged blood clean and then return it to the patient. This would remove the need to transfuse blood from young people, as your own blood could be filtered and returned to you, and no immune reaction either, right?
Irina: Accurate.
This plasmapheresis technique is already approved by the FDA, we believe, so this should help you to develop your project faster, right?
Irina: Exactly.
Do you think a small molecule approach is a viable and, more importantly, a logistically practical approach to calibrate all these factors compared to filtering aged blood?
Irina: Yes, it is a very feasible alternative to the NextGen apheresis that we are working and publishing on.
It is thought that altered signaling is caused by other aging hallmarks higher up in the chain of events; even if we can “scrub” aged blood clean, is it likely to have a long-lasting effect, or would the factors reach pro-aging levels fairly quickly again if nothing is done about the other hallmarks antagonizing them?
Irina: That needs to be established experimentally, but due to the many feedback loops at the levels of proteins, genes and epigenetics, the acquired youthful state might persist.
Ultimately, could a wearable or an implanted device that constantly filters the blood be the solution to these quickly accumulating factors?
Irina: Maybe, but the first step of a day at a NextGen apheresis clinic once every few months might be more realistic.
Filtering seems to be a far more practical solution, so how are you progressing on the road to clinical trials?
Irina: We are collaborating with Dr. Dobri Kiprov, who is a practicing blood apheresis physician with 35 years of experience, and he is interested in repositioning this treatment for alleviating age-related illnesses.
Senolytics and removing senescent cells and the resulting inflammation they cause during the aging process has become a hot topic in the last year or so. What are your thoughts on senolytics as a potential co-therapy with a blood filtering approach?
Irina: Might be good, but we should be careful, as p16 is a normal, good gene that is needed for many productive activities by many cells.
What do you think it will take for the government to fully support the push to develop rejuvenation biotechnology?
Irina: Clear understanding of the current progress and separating the real science from snake oil is very important for guiding funding toward realistic clinical translation and away from the myth and hype.
The field is making amazing progress, but, sadly, it is plagued by snake oil. As much as an “anti-aging free market” encourages innovation, it also encourages hucksters. How can a member of the public tell the difference between credible science and snake oil?
Irina: I was thinking for some time about starting a popularized journal club webpage where ordinary people can see what we typically critically point out in the lab setting about published papers and clinical trials.
How can our readers learn more about your work and support your research?
Irina: The new Conboy lab website is coming up; meanwhile, contact me and Dr. Mike at iconboy@berkeley.edu and conboymj@berkeley.edu
Conclusion
We would like to thank Irina and Michael for taking the time to answer our questions and for providing the readers with a fascinating insight into their work.
Literature
[1] Conboy, I. M., Conboy, M. J., Smythe, G. M., & Rando, T. A. (2003). Notch-mediated restoration of regenerative potential to aged muscle. Science, 302(5650), 1575-1577.
[2] Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., & Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature, 433(7027), 760-764.
[3] Yousef, H., Conboy, M. J., Morgenthaler, A., Schlesinger, C., Bugaj, L., Paliwal, P., … & Schaffer, D. (2015). Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget, 6(14), 11959.
[4] Rebo, J., Mehdipour, M., Gathwala, R., Causey, K., Liu, Y., Conboy, M. J., & Conboy, I. M. (2016). A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nature communications, 7.
[5] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[6] Liu, A., Guo, E., Yang, J., Yang, Y., Liu, S., Jiang, X., … & Gewirtz, D. A. (2017). Young plasma reverses age‐dependent alterations in hepatic function through the restoration of autophagy. Aging cell.
[7] Rebo, J., Mehdipour, M., Gathwala, R., Causey, K., Liu, Y., Conboy, M. J., & Conboy, I. M. (2016). A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nature communications, 7.
[8] Yousef, H., Conboy, M. J., Morgenthaler, A., Schlesinger, C., Bugaj, L., Paliwal, P., … & Schaffer, D. (2015). Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget, 6(14), 11959.