As we age, our bodily functions begin to deteriorate. To some extent, our bodies can cope with these unwelcome changes, but after age 35, some of them become visible. For us living in a world where youth and physical attractiveness are considered an advantage, this gradual loss of young looks can be painful – or maybe even scary, if we don’t know a way to slow down or reverse it.
It is not that physical attractiveness is a value per se for me, but I often hear people say that someone promoting longevity technologies should set a good example; wrinkles, dull skin and hair, and a bloated figure discredit not only the activist but the movement as a whole.
So, I keep an eye on what is going on in the field of aesthetic medicine – especially when it comes close to and crosses with rejuvenation biotechnologies. Last week, I went to one of the flagship research organizations in Moscow – the Human Stem Cells Institute – to interview Dr. Vadim Zorin, the head of the SPRS-therapy project and the developer of a unique approach to skin rejuvenation.
Vadim, thank you very much for finding time to tell our readers about your work. Our community is interested in various approaches to slowing down the aging process. If we recall the Hallmarks of Aging published in 2013, aging of the skin (and other tissues, I suppose), is due to three mechanisms of aging: depletion of the stem cell pool, dysregulation of proteostasis (that is, the cell produces fewer proteins necessary for its normal function, or these proteins are deformed), and cellular aging. Tell us, please, how does SPRS therapy counteract these aging mechanisms?
SPRS-therapy® stands for Service for Personal Regeneration of Skin. It is a combination of personalized medical and diagnostic procedures for skin regeneration when it already carries some signs of aging and other structural changes. SPRS-therapy® is based on the technology of extracting, assessing, cultivating and using autologous (the patient’s own) fibroblasts. We have patented this technology in Russia, the European Union and the United States.
Fibroblasts are the main cellular component of the skin’s connective tissue, maintaining its homeostasis and morphofunctional organization. They perform a number of diverse and complex functions in the skin; they control the composition and structure of the components of the extracellular matrix of the dermis (collagen, elastin, proteoglycans and structural glycoproteins), and their function includes both the production of these substances and their catabolism.
Thus, fibroblasts are a key link in skin biology; they support the homeostasis of the extracellular matrix of the dermis, providing its remodeling and renewal, and they play a significant role in maintaining the physiological state of the other layers of the skin.
 As the population of skin fibroblasts ages, the number of cells decreases in the skin of old people. The total number of fibroblasts is reduced by an average of 35%, and their biosynthetic activity decreases. According to G. Fisher et al., 2002, the production of collagen in the skin of old people is, on average, 75% below that of young people. The balance between the processes of synthesis and degradation of the extracellular matrix of the dermis is disturbed. The natural consequence of these processes is a decrease in the thickness and elasticity of the skin and the formation of wrinkles. Hence, the process of skin aging is ultimately caused by a decrease in the fibroblast population and a decrease in their proliferative/synthetic activity, which is naturally manifested by a decrease in the quantitative and qualitative composition of the extracellular matrix of the dermis.
As the population of skin fibroblasts ages, the number of cells decreases in the skin of old people. The total number of fibroblasts is reduced by an average of 35%, and their biosynthetic activity decreases. According to G. Fisher et al., 2002, the production of collagen in the skin of old people is, on average, 75% below that of young people. The balance between the processes of synthesis and degradation of the extracellular matrix of the dermis is disturbed. The natural consequence of these processes is a decrease in the thickness and elasticity of the skin and the formation of wrinkles. Hence, the process of skin aging is ultimately caused by a decrease in the fibroblast population and a decrease in their proliferative/synthetic activity, which is naturally manifested by a decrease in the quantitative and qualitative composition of the extracellular matrix of the dermis.
So, the loss of functional fibroblasts seems to be the key enemy of those of us chasing indefinite youth, at least on the outside. How exactly does SPRS-therapy fight against these unwelcome, age-related changes?
The use of cultured autologous dermal fibroblasts (autoDF) allows us to replenish the decreased fibroblast population by introducing specialized, functionally active cells into the skin of the patient.
As early as 1994, American scientists showed that the introduction of autoDF into the skin promotes effective wrinkle correction, and a number of clinical studies have been carried out by both American and Russian scientists to confirm the effectiveness and safety of autoDF in cosmetic medicine.
Building upon this work, we developed our own method to fence, transport, isolate, cultivate, cryopreserve, store and apply autologous fibroblasts for skin rejuvenation. We took it into clinical trials and proved its positive effect. In 2010, the Human Stem Cells Institute (HSCI) in Russia received permission from the Russian Federal Service for Surveillance in the Health Care Sector to use SPRS-therapy to treat age-related and cicatricial changes in the skin. In 2011, the FDA in the United States issued a permit to another company, Fibrocell Science, to use autoDF to correct wrinkles in the area of nasolabial folds (it is called LAVIVTM, or azficel-T).
 Through instrumental and morphological studies, we observed an increase in the thickness and elasticity of the skin and a decrease in the number and depth of wrinkles. This indicates that after the transplantation, cultured autoDF cells are fully integrated into the dermis, and their biosynthetic activity persists for at least 12 months. As a result, the microstructure of the dermis is remodeled, increasing its collagen fibers and thus increasing skin hydration, density, and thickness. This particular clinical effect becomes more pronounced throughout the year after the intervention, and it lasts for at least two years. Our results are consistent with the results of research conducted by the American company Fibrocell Science, which also demonstrated a significant decrease in the number of wrinkles and increased skin thickness after the use of autoDF.
Through instrumental and morphological studies, we observed an increase in the thickness and elasticity of the skin and a decrease in the number and depth of wrinkles. This indicates that after the transplantation, cultured autoDF cells are fully integrated into the dermis, and their biosynthetic activity persists for at least 12 months. As a result, the microstructure of the dermis is remodeled, increasing its collagen fibers and thus increasing skin hydration, density, and thickness. This particular clinical effect becomes more pronounced throughout the year after the intervention, and it lasts for at least two years. Our results are consistent with the results of research conducted by the American company Fibrocell Science, which also demonstrated a significant decrease in the number of wrinkles and increased skin thickness after the use of autoDF.
Sounds pretty amazing! Of course, someone who is serious about restoring a youthful appearance won’t use just one therapy but rather a combination. This includes plastic surgery and other injection methods, such as the recently popular PRP or fillers. However, your therapy has been clinically tested, so its effect was monitored. What did your patients say, and how many years younger did they look after the course of treatment? How exactly do you measure the results?
It is difficult to say how much younger it is after SPRS-therapy, since such studies have not yet been conducted by anyone, but we can definitively measure changes in the metrics that are “responsible” for the youth and beauty of skin, including its thickness, density and hydration, intensity of pigment spots, and number and depth of wrinkles. Our clinical analysis of the skin’s condition (on a 5-point scale) showed that one month after the injection, 88% of the patients rated the result as “good” and “excellent”, but after 3, 6, 12, and 24 months, it was 100% of patients.
We performed histological studies; simultaneously with the introduction of the autoDF into the skin, we injected our participants in a spot behind the auricle for subsequent biopsies at 1, 3, 6, 12 and 24 months. We conducted morphometric evaluation of the thickness of the dermis and impregnated it with silver nitrate in order to detect newly formed collagen fibers. Our immunohistochemistry studies revealed prolonged biosynthetic activity of transplanted autoDF for at least 12 months, which was expressed in the synthesis of components of the cellular matrix and an increase in the thickness of the dermis by an average of 63% over those 12 months. We also evaluated microcirculation with laser Doppler flowmetry (laser blood flow analyzer), elasticity with cutometry (Cutometer MPA 580, Courage + Khazaka Electronic GmbH), skin texture and wrinkles using the VISIA photometric system (Proctor & Gamble Co), and wrinkle depth by means of optical profilometry (PRIMOS, GFMesstechnik GmbH).
These measurements showed a significant increase in the elasticity and thickness of the skin, a decrease in the intensity of pigment spots, and a decrease in the number and depth of wrinkles.
It is wonderful that we have a way to achieve external rejuvenation. Moreover, this affects not only the condition of the skin but also the common problem of hair loss. As I understand it, this is especially important for men; what is the proportion of the male population among your patients?
About one-third of our patients are men.
Is it possible to restore the pigmentation of hair in people who have already turned gray?
 To date, no, it’s not currently possible. This is a very complex process, since physiological graying is associated with the natural aging of other cells, specifically melanocytes, and early graying, as a rule, is due to their death or decreased activity due to hormonal disorders. As a result, the hair is deprived of the pigment, it acquires a porous structure, and the air between the layers gives the affected hair a silvery white hue. We cannot stop the process of graying because melanocytes begin to work even in the prenatal period of human development and gradually regress with age. Every 10 years after the age of 30, their function fades by 10-20% and hair begins to appear with no pigment in the keratin. With the withering away of all the melanocytes supplying melanin to the hair shaft, the hair becomes completely gray.
To date, no, it’s not currently possible. This is a very complex process, since physiological graying is associated with the natural aging of other cells, specifically melanocytes, and early graying, as a rule, is due to their death or decreased activity due to hormonal disorders. As a result, the hair is deprived of the pigment, it acquires a porous structure, and the air between the layers gives the affected hair a silvery white hue. We cannot stop the process of graying because melanocytes begin to work even in the prenatal period of human development and gradually regress with age. Every 10 years after the age of 30, their function fades by 10-20% and hair begins to appear with no pigment in the keratin. With the withering away of all the melanocytes supplying melanin to the hair shaft, the hair becomes completely gray.
A more difficult question is whether this approach is applicable to the restoration of other tissues and organs, or does SPRS only allow you to restore the skin? If there are restrictions, then what are they related to?
SPRS-therapy is aimed at restoring only the skin because it is based on the use of skin fibroblasts. However, in the arsenal of our company, there is another authorized technology: SPRG-therapy (patented in Russia in 2010), which is based on the use of fibroblasts of the oral mucosa, which allows the restoration of soft periodontal tissue. So, we can say that this technology can be adapted to some of other cell types, but each new type requires its own set of production processes and, of course, clinical studies.
Last year, our community was enthused by news from the Salk Institute, where due to reprogramming of adult cells with Yamanaka factors, the researchers managed to partially reverse some age-related changes in mice, such as muscular dystrophy or age-related metabolic disorders. What do you think about the combination of SPRS and the short-term application of Yamanaka factors on cell culture? Can the further development of SPRS therapy go in this direction?
 Yes, of course, the reprogramming of autologous skin fibroblasts will allow obtaining personalized induced pluripotent cells (iPSCs), which can be considered innovations towards autogenous therapy of a wide range of diseases, and iPSCs are also useful for drug screening. However, it should be noted that, despite the promising therapy options based on autologous iPSCs, this technology is still at an early stage of development. Further research is needed, including a detailed study of the biological and cancer-related safety of these cells, an analysis of their chromosomal stability both in early and late passages, and a complex analysis of their differentiation potential.
Yes, of course, the reprogramming of autologous skin fibroblasts will allow obtaining personalized induced pluripotent cells (iPSCs), which can be considered innovations towards autogenous therapy of a wide range of diseases, and iPSCs are also useful for drug screening. However, it should be noted that, despite the promising therapy options based on autologous iPSCs, this technology is still at an early stage of development. Further research is needed, including a detailed study of the biological and cancer-related safety of these cells, an analysis of their chromosomal stability both in early and late passages, and a complex analysis of their differentiation potential.
At the moment, SPRS therapy is quite expensive; the price for one course of treatment can be more than $5,000. For this anti-aging approach to become available to the masses, the price should be significantly reduced. HSCI has a very positive history with regards to reducing the price of an innovative product. I’m talking about gene therapy, which is developed at the institute. Your Neovasculgen is designed to stimulate vascular growth in a limb in order to replace vessels lost due to severe limb ischaemia. Over the past couple of years, you have been able to reduce the price of Neovasculgen more than two-fold, and now it costs around $1,500 – probably the most affordable gene therapy in the world. It was a hard decision for you of course, as this price can barely recoup what you invested into the research behind it, but it allowed you to have this gene therapy included in the list of essential medicines in Russia, which improves access to it. Can we expect similar breakthroughs in the case of SPRS?
It is necessary to understand that in the case of SPRS therapy, we do not work directly with patients; the final price for the patient is set by the clinics, with which we work in terms of bilateral agreements. Quite often, to our great regret, the final price is triple our production cost (i.е. what we spend to produce the cell culture for one individual patient), and in some clinics, it is quadruple or quintuple! Now, we are trying to work with new clinics to reduce their costs so that we can make this therapy affordable for more people.
In addition to helping people to become younger, you often write in your blog about your personal health strategy. What elements, in your opinion, should be the basis of such a strategy? What methods of slowing aging do you consider to be sufficiently scientifically valid, and what are you personally using?
What happens to us with increasing age? First, an increase in food intake; second, a decrease in motor activity; and third, a decrease in the body’s ability to mobilize fats. Starting at age 30, alas, the fat content in the body begins to increase, but the net body weight – due to the reduction of muscle tissue and demineralization of bone tissue – begins to decrease. It is caused by many factors, not least of which is the same decrease in motor activity due to metabolic processes deteriorating with age. This becomes a vicious cycle. Therefore, the algorithm is very simple.
1) Increased physical activity, which leads to greater energy expenditure. What kind of training is the easiest and most useful? Ordinary walking or, better yet, Nordic walking in the cardiological step (ideally 5-6 km/h with a pulse rate of 120 beats per minute). This is a training in the so-called aerobic zone, i.e. low-intensity and long-term physical activity.
 I am an adherent of Nordic walking, which employs up to 85% of the muscles of the body and, in addition, supports energy metabolism, pulmonary ventilation and muscle tone, which plays an important role in preventing the development of ischaemic heart disease. However, since any physical activity should be appropriate for age and health status, it is mandatory to control the pulse (heart rate). To monitor my pulse, distance and speed in real time, I use the Runtastic PRO program installed on my mobile phone. My standard training is to walk for at least 3 times a week for a minimum of 30 to 40 minutes. Incidentally, exercise not only strengthens and increases muscle and bone tissue (and we have neither more nor less than 206 bones and more than 640 muscles), it also stimulates the activity of stem cells that are responsible for maintaining the number of cells inhabiting their tissues.
I am an adherent of Nordic walking, which employs up to 85% of the muscles of the body and, in addition, supports energy metabolism, pulmonary ventilation and muscle tone, which plays an important role in preventing the development of ischaemic heart disease. However, since any physical activity should be appropriate for age and health status, it is mandatory to control the pulse (heart rate). To monitor my pulse, distance and speed in real time, I use the Runtastic PRO program installed on my mobile phone. My standard training is to walk for at least 3 times a week for a minimum of 30 to 40 minutes. Incidentally, exercise not only strengthens and increases muscle and bone tissue (and we have neither more nor less than 206 bones and more than 640 muscles), it also stimulates the activity of stem cells that are responsible for maintaining the number of cells inhabiting their tissues.
2) Deliberate diet control. Calorie restriction prolongs life in any organism from yeast to primates, including humans. In 2007, evidence of this phenomenon appeared at the cellular level when American scientists, led by Dr. Sinclair (published in the prestigious scientific journal Cell), discovered the genes SIRT3 and SIRT4. The activation of these genes leads to an increase in the same proteins as the family of sirtuins, which are important regulators of slowing the aging of cells and increasing longevity. In general terms, this is the case. There are “power plants” – mitochondria – in the cytoplasm of our cells. When the calories entering the cell are reduced, relevant signals enter the mitochondria and activate a specific NAMPT gene in them, which leads to an increase in the production of certain molecules (the so-called NAD – the main energy carriers in the cell), which, in turn, activate the SIRT3 and SIRT4 genes. The proteins expressed by these genes lead to an improvement in energy metabolism in the cell, and this, in turn, leads to a slower aging of the cell and prevents apoptosis (premature cell death).
As a result, the body slows down the aging process, the development of age-related diseases is delayed, and, accordingly, the lifespan is increased.
This mechanism is proved by the results of a 25-year study published in Nature Communications in 2014. This study was conducted by a team of scientists from the University of Wisconsin-Madison on rhesus macaques. Macaques on a low-calorie diet had normal body weight, a decrease in the rate of loss of muscle mass (which, as is known, accelerates with age), a significant reduction in the risk of developing diabetes and cardiovascular diseases, and an increased lifespan of 30 years or more, despite the fact that the average life expectancy in captivity for these animals is about 26. Incidentally, Sinclair and colleagues assert that exercise exerts the same effect on the body as a low-calorie diet!
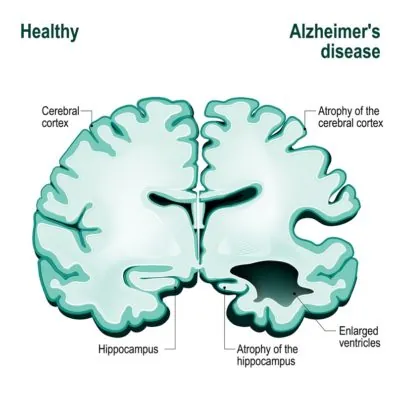
3) And, of course, do not forget about the sauna, unless it is contraindicated for health reasons. A sauna offers relaxation, dilates blood vessels, and may reduce the risk of developing respiratory and cardiovascular diseases, senile dementia and even Alzheimer’s disease!
We often ask researchers what their forecast is regarding medical technologies for addressing aging. Do you think that science will be able to defeat aging and thereby prevent age-dependent diseases? What can we do as a community to make this happen sooner?
I think that someday in the future it will happen. It may not be exactly defeating aging but rather increasing the life expectancy and significantly improving the quality of life. However, a lot has already been done in this respect. Which directions do I find promising? Here they are:
1) Gene therapy. In the laboratory, we have already managed to prolong the life of nematode worms, fruit flies, and mice. In humans, genes responsible for prolonging life have also been identified.
2) Cell therapy. Today, a lot of clinical studies are conducted on the treatment of so-called classical age-related diseases by introducing allogenic mesenchymal stromal cells (MSCs) into the damaged parts of the brain and heart, which significantly improves the condition of patients who have suffered strokes and heart attacks. In 2017, a randomized, blind, placebo-controlled clinical trial was conducted in 30 patients with senility syndrome, who were intravenously injected with allogeneic MSCs from young healthy donors. The results of the study showed a significant improvement in physical health in these patients as well as a decrease in the content of inflammatory biomarkers that are specific to this syndrome.
With the help of cell therapy, good results have also been obtained in the restoration of bones, joints, and skin. At present, many leading laboratories in the world have made considerable progress in studying induced pluripotent cells (iPSCs). These cells are desirable because, on the one hand, they have the properties of embryonic stem cells (ESCs) that make them capable of differentiating into any type of cell in the body, and on the other hand, their use makes it possible to avoid the ethical and other problems associated with ESCs. These cells enable the creation of innovative technologies for autogenous therapy of a wide range of diseases, including, of course, age-related pathologies.
3) Growing and transplanting organs. We have already learned to grow in laboratories not only cartilaginous tissue, skin, and blood vessels but also the ureter, bladder, and other hollow organs. The researchers are also working on the creation of artificial hearts and lungs. In short, the prospects are very bright.
What can we do as a society? Increase education and put a maximum of effort into supporting the progress of science and medicine.
I cannot agree more. Thank you very much for your time, Vadim! We wish you and your wife and research partner Alla good luck in further studies!






 Written in an elegant, clear style,
Written in an elegant, clear style, 

 As the population of skin fibroblasts ages,
As the population of skin fibroblasts ages,  Through instrumental and morphological studies, we observed an increase in the thickness and elasticity of the skin and a decrease in the number and depth of wrinkles. This indicates that after the transplantation, cultured autoDF cells are fully integrated into the dermis, and their biosynthetic activity persists for at least 12 months. As a result, the microstructure of the dermis is remodeled, increasing its collagen fibers and thus increasing skin hydration, density, and thickness. This particular clinical effect becomes more pronounced throughout the year after the intervention, and it lasts for at least two years. Our results are consistent with the results of research conducted by the American company Fibrocell Science, which also demonstrated a significant decrease in the number of wrinkles and increased skin thickness after the use of autoDF.
Through instrumental and morphological studies, we observed an increase in the thickness and elasticity of the skin and a decrease in the number and depth of wrinkles. This indicates that after the transplantation, cultured autoDF cells are fully integrated into the dermis, and their biosynthetic activity persists for at least 12 months. As a result, the microstructure of the dermis is remodeled, increasing its collagen fibers and thus increasing skin hydration, density, and thickness. This particular clinical effect becomes more pronounced throughout the year after the intervention, and it lasts for at least two years. Our results are consistent with the results of research conducted by the American company Fibrocell Science, which also demonstrated a significant decrease in the number of wrinkles and increased skin thickness after the use of autoDF. To date, no, it’s not currently possible. This is a very complex process, since physiological graying is associated with the natural aging of other cells, specifically melanocytes, and early graying, as a rule, is due to their death or decreased activity due to hormonal disorders. As a result, the hair is deprived of the pigment, it acquires a porous structure, and the air between the layers gives the affected hair a silvery white hue. We cannot stop the process of graying because melanocytes begin to work even in the prenatal period of human development and gradually regress with age. Every 10 years after the age of 30, their function fades by 10-20% and hair begins to appear with no pigment in the keratin. With the withering away of all the melanocytes supplying melanin to the hair shaft, the hair becomes completely gray.
To date, no, it’s not currently possible. This is a very complex process, since physiological graying is associated with the natural aging of other cells, specifically melanocytes, and early graying, as a rule, is due to their death or decreased activity due to hormonal disorders. As a result, the hair is deprived of the pigment, it acquires a porous structure, and the air between the layers gives the affected hair a silvery white hue. We cannot stop the process of graying because melanocytes begin to work even in the prenatal period of human development and gradually regress with age. Every 10 years after the age of 30, their function fades by 10-20% and hair begins to appear with no pigment in the keratin. With the withering away of all the melanocytes supplying melanin to the hair shaft, the hair becomes completely gray. Yes, of course, the reprogramming of autologous skin fibroblasts will allow obtaining personalized induced pluripotent cells (iPSCs), which can be considered innovations towards autogenous therapy of a wide range of diseases, and iPSCs are also useful for drug screening. However, it should be noted that, despite the promising therapy options based on autologous iPSCs, this technology is still at an early stage of development. Further research is needed, including a detailed study of the biological and cancer-related safety of these cells, an analysis of their chromosomal stability both in early and late passages, and a complex analysis of their differentiation potential.
Yes, of course, the reprogramming of autologous skin fibroblasts will allow obtaining personalized induced pluripotent cells (iPSCs), which can be considered innovations towards autogenous therapy of a wide range of diseases, and iPSCs are also useful for drug screening. However, it should be noted that, despite the promising therapy options based on autologous iPSCs, this technology is still at an early stage of development. Further research is needed, including a detailed study of the biological and cancer-related safety of these cells, an analysis of their chromosomal stability both in early and late passages, and a complex analysis of their differentiation potential. I am an adherent of Nordic walking, which employs up to 85% of the muscles of the body and, in addition, supports energy metabolism, pulmonary ventilation and muscle tone, which plays an important role in preventing the development of ischaemic heart disease. However, since any physical activity should be appropriate for age and health status, it is mandatory to control the pulse (heart rate). To monitor my pulse, distance and speed in real time, I use the Runtastic PRO program installed on my mobile phone. My standard training is to walk for at least 3 times a week for a minimum of 30 to 40 minutes. Incidentally, exercise not only strengthens and increases muscle and bone tissue (and we have neither more nor less than 206 bones and more than 640 muscles), it also stimulates the activity of stem cells that are responsible for maintaining the number of cells inhabiting their tissues.
I am an adherent of Nordic walking, which employs up to 85% of the muscles of the body and, in addition, supports energy metabolism, pulmonary ventilation and muscle tone, which plays an important role in preventing the development of ischaemic heart disease. However, since any physical activity should be appropriate for age and health status, it is mandatory to control the pulse (heart rate). To monitor my pulse, distance and speed in real time, I use the Runtastic PRO program installed on my mobile phone. My standard training is to walk for at least 3 times a week for a minimum of 30 to 40 minutes. Incidentally, exercise not only strengthens and increases muscle and bone tissue (and we have neither more nor less than 206 bones and more than 640 muscles), it also stimulates the activity of stem cells that are responsible for maintaining the number of cells inhabiting their tissues.