Researchers publishing in Nature Aging have discovered that extracellular vesicles (EVs) derived from antler blastema progenitor cells (ABPCs) restore bone mass to rhesus macaques.
Finding the most effective EVs
In the world of rejuvenation research, EVs are nothing new. We have covered them extensively, as studies have repeatedly found benefits for the heart and effectiveness against cellular senescence. Because they are derived from stem cells rather than being cells themselves, there are no concerns with immunorejection [1].
Deer antlers are the only organ that regenerates fully in adulthood, and so antler cells have been an attractive source of pro-regeneration EVs. One study found that ABPCs remain robust even after 50 cellular cycles and that their EVs are a potential treatment for arthritis [2]. These researchers have previously found a population of ABPCs with particularly strong potential [3]; this study uses those cells’ EVs against multiple age-related targets.
More effective than bone marrow cells
In their first experiment, the researchers compared their ABPCs to bone marrow stem cells (BMSCs) derived from aged and fetal rats. The ABPCs proliferated far faster than either BMSC group, growing at a rate nearly six times that of the adult cells and over three times as fast as the fetal cells. They had significantly lower senescence markers as well. These differences were due to cell type rather than species; BMSCs derived from young male deer had roughly the same proliferation rates as fetal rat BMSCs.
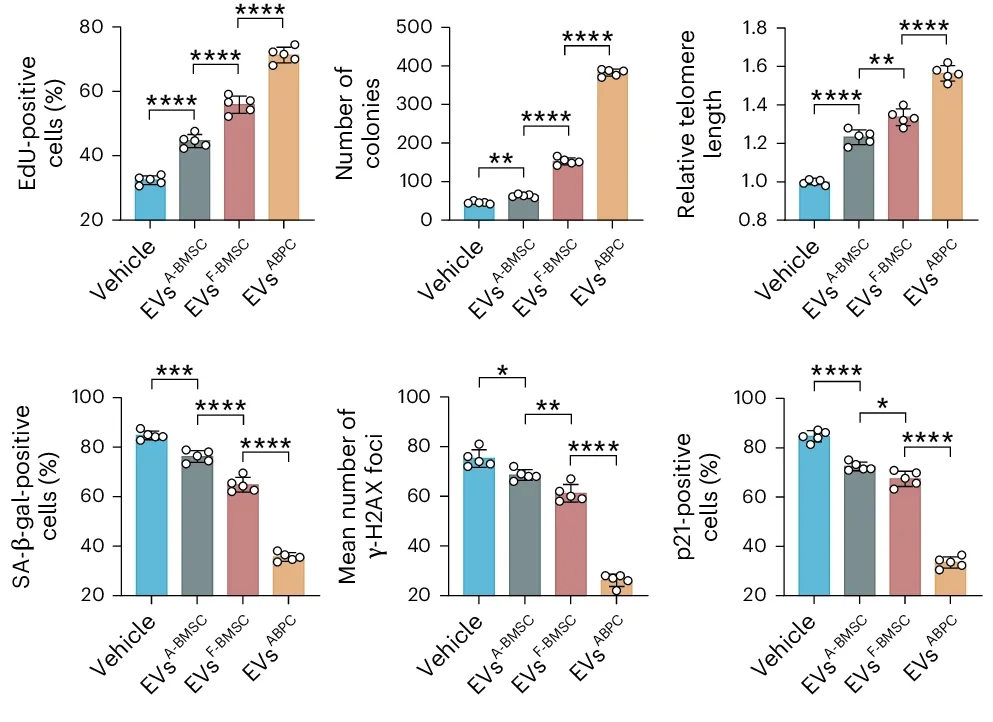
ABPCs also produced more EVs than BMSCs, producing almost ten times as many as adult BMSCs and twice as many as fetal BMSCs. An RNA analysis of ABPC-derived EVs found many beneficial factors, including directly pro-regeneration factors along with maintenance of the cell cycle, telomere length and proteostasis. Inflammatory factors were downregulated in these EVs, and they were better than fetal BMSCs at promoting cellular function.
ABPC-derived EVs were more effective than fetal BMSC-derived EVs in attenuating aging in adult BMSCs. Compared to the fetal group, the ABPC group had far less of the DNA damage marker γ-H2AX, greatly reduced senescence markers, and significantly more proliferation. Additionally, aging causes BMSCs to shift from bone to fat, a cause of age-related bone loss; ABPC-derived EVs were more effective than BMSC-derived EVs in reversing this tendency.
The researchers identified a single, crucial mRNA in EVs that was responsible for a substantial portion of this effect: Prkar2a, which is involved in the cell cycle and in cellular development. Adult ABPCs that had impaired response to Prkar2a received far fewer benefits.
Effective in mice
The researchers then administered the various EVs to aged mice for four weeks. Here, again, ABPCs outperformed the other groups, substantially increasing bone strength and mineral density. While osteoclasts, which degrade bone, were similar among all the tested groups, the ABPC group had much more osteoblast activity, which represents more bone formation.
Once more, isolating Prkar2a had substantial benefits: removing it from ABPC-derived EVs significantly diminished their effectiveness, while adding it to adult BMSC-derived EVs greatly enhanced theirs.
There were also substantial systemic benefits from ABPC-derived EV administration. Mice given these EVs had better balance and less fatigue along with a reduction in SASP-related inflammatory markers such as interleukins. Gene expression related to oxidative stress was reduced, and epigenetic aging markers showed evidence of rejuvenation in this area as well.
There were multiple benefits across multiple organs. The ABPC EV-treated mice’s kidneys had less evidence of cellular death by apoptosis, and their livers and kidneys had less fibrosis. There was also a neuroprotective effect, as these mice’s brains had less evidence of DNA damage. Systemic benefits were found in both male and female mice.
The effects on the brain were examined more closely, and the researchers found evidence of better brain function; the treated mice were more interested in new things, as measured by the Y maze test and the novel object recognition test. They also spent more time in the open arms in an elevated maze, suggesting reduced anxiety.
Monkeys received benefits as well
Because of their similarity to human beings, non-human primates, such as the rhesus macaques used in this study, are commonly used to test the effectiveness of promising approaches. Compared to a control group that only received saline, treating older female monkeys with ABPC-derived EVs greatly increased their willingness to move without affecting their ability to sleep. Improvements in motor dexterity were also noted.
The benefits to cellular function found in mice were also observed in these monkeys. Prkar2a was upregulated, inflammatory cytokines and senescent cells were reduced, and epigenetic age was reduced in bone marrow. While the monkeys’ intelligence was not analyzed, brain imaging found substantial improvements to their grey matter, including in the cerebral cortex.
While the researchers do not yet recommend their ABPC-derived EVs for human use, suggesting that there may be a possibility of tumors over the long term, they note that their findings provide a foundation for potential treatments. It may be possible to isolate the key factors that make ABPC-derived EVs so effective, such as Prkar2a, and administer them directly.
Literature
[1] Zhang, K., & Cheng, K. (2023). Stem cell-derived exosome versus stem cell therapy. Nature Reviews Bioengineering, 1(9), 608-609.
[2] Lei, J., Jiang, X., Li, W., Ren, J., Wang, D., Ji, Z., … & Wang, S. (2022). Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein & cell, 13(3), 220-226.
[3] Qin, T., Zhang, G., Zheng, Y., Li, S., Yuan, Y., Li, Q., … & Qiu, Q. (2023). A population of stem cells with strong regenerative potential discovered in deer antlers. Science, 379(6634), 840-847.


