In Cell Stem Cell, researchers have published the results of a Phase 1 clinical trial of a treatment that uses neural stem cells (NSCs) to treat multiple sclerosis.
Looking for better therapies
Multiple sclerosis (MS) is a progressive disease that afflicts approximately two million people worldwide [1]. This is an autoimmune disease that causes bursts of inflammation, strips the myelin from neurons, and causes their gradual decline and death [2]. The disease, which often manifests in young adulthood, takes approximately a quarter century to progress to lasting disability for which there is no effective treatment [3]. Existing therapies have meaningful effects but cannot reverse the progression of the disease.
In animal studies, stem cells have shown promise in potentially treating MS. MS model rats given rat NSCs had those cells proliferate and differentiate, restoring cell population and function [4]. Better function and less inflammation were found in an MS-model primate study in which human NSCs were used [5].
The cells used in this study were not generated from the patients’ own cells; rather, they were allogeneic cells derived from a stable cell line derived from a single donor. In mice, these cells reproduced rapidly into the cells needed for brain function, including astrocytes and neurons. With those preclinical successes in hand, these researchers began to test their approach: directly injecting NSCs into the brains of human beings.
Straight to the brain
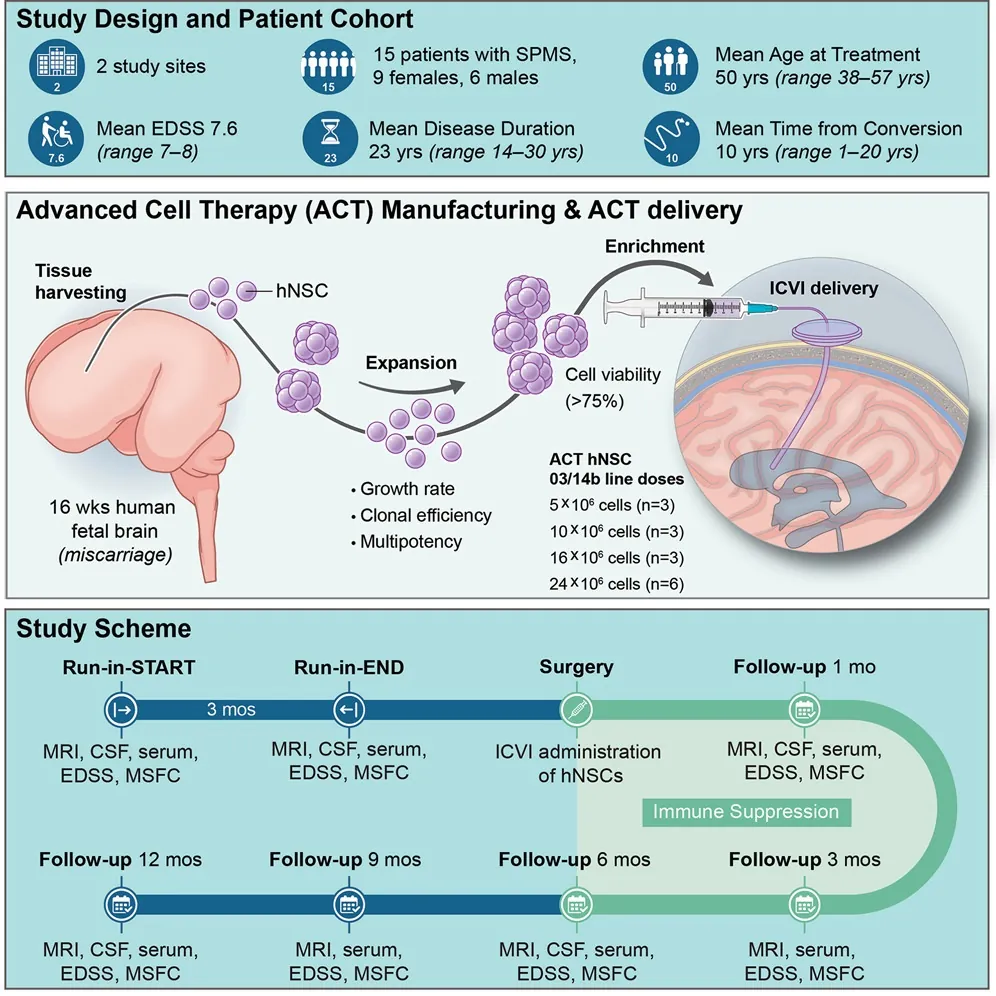
Out of 180 potential candidates, 15 volunteers were chosen for this treatment, with doses ranging from 5 million cells to 24 million NSCs. A catheter was placed in each of the patients’ brains, after which the cells were funneled directly into the central brains of each patient.
Over the year of monitoring after injection, none of the treated patients got significantly worse in function: disability remained more or less constant, neither progressing nor receding. The researchers caution that, due to the severe disability of these volunteers, measurements are difficult to conduct. There were no serious adverse events nor deaths in the patients.
The patients received regular MRIs to evaluate changes to their brains. There were no statistically significant changes in brain lesions over the course of the study. However, there was a dose-dependent relationship between the amount of stem cells injected and the amount of brain volume retained: people with larger doses retained more of their brains. The researchers speculate, but cannot prove, that this may be due to decreased inflammation.
Many caveats
This was a Phase 1 clinical trial, which had no control group and was geared towards testing safety rather than effectiveness. Each case of MS was different, and there was a low sample size and a wide variety of doses. The researchers note that, as immunosuppression was a required part of the study protocol, it is possible that undetected inflammatory effects were occurring as a result of the treatment.
While this study did not demonstrate therapeutic efficacy, it did demonstrate that injecting stem cells directly into people’s brains can be conducted feasibly and safely. Future trials may use NSC treatments for such ailments as Parkinson’s, Alzheimer’s, and possibly age-related cognitive decline. Such future approaches may also make it possible to use methods that do not require immunosuppressants.
Literature
[1] Stenager, E. (2019). A global perspective on the burden of multiple sclerosis. The Lancet Neurology, 18(3), 227-228.
[2] Dobson, R., & Giovannoni, G. (2019). Multiple sclerosis–a review. European journal of neurology, 26(1), 27-40.
[3] Confavreux, C., & Vukusic, S. (2014). The clinical course of multiple sclerosis. Handbook of clinical neurology, 122, 343-369.
[4] Pluchino, S., Quattrini, A., Brambilla, E., Gritti, A., Salani, G., Dina, G., … & Martino, G. (2003). Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature, 422(6933), 688-694.
[5] Pluchino, S., Gritti, A., Blezer, E., Amadio, S., Brambilla, E., Borsellino, G., … & Martino, G. (2009). Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 66(3), 343-354.