In Nature Biomedical Engineering, a team of researchers has published an innovative method of making tau tangles and amyloid beta visible in the near infrared, allowing doctors and researchers to see through bone with relatively simple equipment.
Current methods are difficult
There are three main approaches to diagnosing Alzheimer’s and other brain disorders in living people: magnetic resonance imaging (MRI) [1], positron emission tomography (PET) [2], and single-photon emission computed tomography (SPECT) [3]. Unfortunately, all three require bulky and expensive equipment. An MRI creates a substantial magnetic field and can’t be safely performed on someone with metallic objects in the body, while both PET and SPECT scans involve radioactive tracers.
It would be ideal if tau tangles and amyloid plaques could simply be viewed optically. While this appears impossible, prior work had explored the idea, developing phosphorescent compounds that attach themselves to the characteristic amyloids of Alzheimer’s [4]. This runs into an obvious problem: how would it be possible to see this glow through the skull and scalp?
While mammalian tissue blocks the visible spectrum, it is much more transparent to near-infrared light. Other researchers have explored this idea, developing phosphorescent proteins for potential use in diagnostics [5]. However, this paper is the first of its kind in developing a method to attach such near-infrared phosphors to Alzheimer’s amyloids in living animals.
An improvement over previous compounds
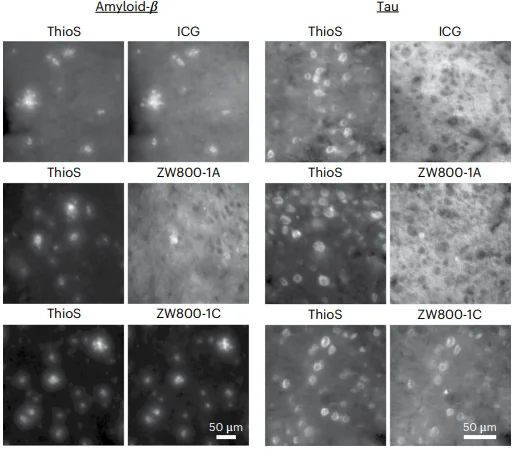
This paper investigates the new compound ZW800-1C, which is structurally similar to the FDA-approved ICG and is closely related to a previously developed compound, ZW800-1A.
In an ex vivo examination, ICG was found to bind almost exactly as well to amyloid beta plaques as ThioS, an established compound that emits violet light. However, in a transgenic mouse model, ICG failed to bind to in vivo plaques and did not bind well to signs of cerebral amyloid angiopathy (CAA), another sign of Alzheimer’s disease. The researchers hypothesize that this is because it failed to cross the blood-brain barrier. It also has a relatively short half-life in blood.
ZW800-IA fared little better as a potential diagnostic. Ex vivo, it did not clearly bind to amyloid at all, and in vivo, its visibility was muted for both plaques and CAA.
ZW800-IC was much more effective. It rapidly bound to amyloid, and it was found to be very visible in ex vivo and in vivo models, including both plaques and CAA. It was better distributed in tissue, lasted longer in blood, and, most importantly, was found to be reactive with tau pathology, a trait not shared by either of the other two compounds.
Fluorescent compounds such as these only emit photons when they are struck by photons; the amount of time between absorption and emission is known as its lifetime, which is measured in picoseconds for ZW800-IC. This lifetime was found to vary between blood vessels, amyloid plaques, CAA, and tau tangles. Additionally, its emission was in different wavelengths depending on its attachment to Aβ40 or Aβ42, two variants of amyloid beta. These distinctions are potentially valuable in diagnosing Alzheimer’s.
Conclusion
This paper concludes with a key observation. As humans are much larger than mice and there is much more tissue to penetrate, using targeted photons to activate fluorescent proteins for microscopically close examination is infeasible. A more scattered, generalized approach would have to be used instead. However, if the lifetime or spectrum of the fluorescence are different, as they have been shown to be in this study, those details could be useful biomarkers for Alzheimer’s pathology and potentially help in the early diagnosis of Alzheimer’s disease.
This approach might also be of considerable use for researchers who are using mouse models to develop therapies against Alzheimer’s and tauopathies. In many cases, examining the brains of living animals through the intact skull, as opposed to cranial windows or euthanasia-based approaches, is likely to save money, time, and animals.
Literature
[1] Poduslo, J. F., Wengenack, T. M., Curran, G. L., Wisniewski, T., Sigurdsson, E. M., Macura, S. I., … & Jack Jr, C. R. (2002). Molecular targeting of Alzheimer’s amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiology of disease, 11(2), 315-329.
[2] Mathis, C. A., Mason, N. S., Lopresti, B. J., & Klunk, W. E. (2012, November). Development of positron emission tomography β-amyloid plaque imaging agents. In Seminars in nuclear medicine (Vol. 42, No. 6, pp. 423-432). WB Saunders.
[3] Valotassiou, V., Malamitsi, J., Papatriantafyllou, J., Dardiotis, E., Tsougos, I., Psimadas, D., … & Georgoulias, P. (2018). SPECT and PET imaging in Alzheimer’s disease. Annals of nuclear medicine, 32, 583-593.
[4] Heo, C. H., Sarkar, A. R., Baik, S. H., Jung, T. S., Kim, J. J., Kang, H., … & Kim, H. M. (2016). A quadrupolar two-photon fluorescent probe for in vivo imaging of amyloid-β plaques. Chemical science, 7(7), 4600-4606.
[5] Shcherbakova, D. M., Stepanenko, O. V., Turoverov, K. K., & Verkhusha, V. V. (2018). Near-infrared fluorescent proteins: multiplexing and optogenetics across scales. Trends in biotechnology, 36(12), 1230-1243.





