Targeting an Inflammatory Pathway Fights Alzheimer’s
- This approach affects tau pathology but not amyloid beta.

- A mutation in the Alzheimer’s-related APOE gene affects a key inflammatory pathway.
- People with this mutation have a unique protection against Alzhimer’s.
- The effects of this mutation may be possible to clinically administer.
Scientists have discovered that a rare mutation protects against Alzheimer’s disease by dampening a central inflammatory pathway. They recapitulated these results using a small molecule [1].
The woman who beat the odds
For the last 40 years, scientists have been studying a massive extended family of about 6,000 people in and around Medellín, Colombia. Many members of this family carry a rare genetic mutation, PSEN1 E280A (Paisa). Its carriers are virtually guaranteed to develop early-onset Alzheimer’s disease. Typically, they show signs of cognitive impairment in their mid-40s, develop dementia in their 50s, and die in their 60s.
One woman in this family, Aliria Rosa Piedrahita de Villegas, was a remarkable exception. Despite carrying the Paisa mutation, she remained cognitively healthy until her early 70s and died from cancer in 2020 at the age of 77.
Aliria’s post-mortem brain scans showed that her brain was full of amyloid plaques, one of the key pathological hallmarks of Alzheimer’s. However, she had very little of the second hallmark, neurofibrillary tangles of tau protein, especially in brain regions related to memory.
It turned out that, in addition to the harmful Paisa mutation, Aliria also had two copies of another, extremely rare mutation in the APOE3 gene. This variant is known as the R136S or Christchurch mutation, named after the city in New Zealand where it was first identified.
Since then, researchers have identified several dozen people with one copy of the mutated gene instead of two, making them heterozygous. In those people, the mutation was mildly protective, hinting at a dose-dependent relationship [2]. However, until now, little has been known about the mechanism behind the protective effect of the Christchurch mutation. A new study from Weill Cornell Medicine provides an interesting answer.
Numerous protective effects
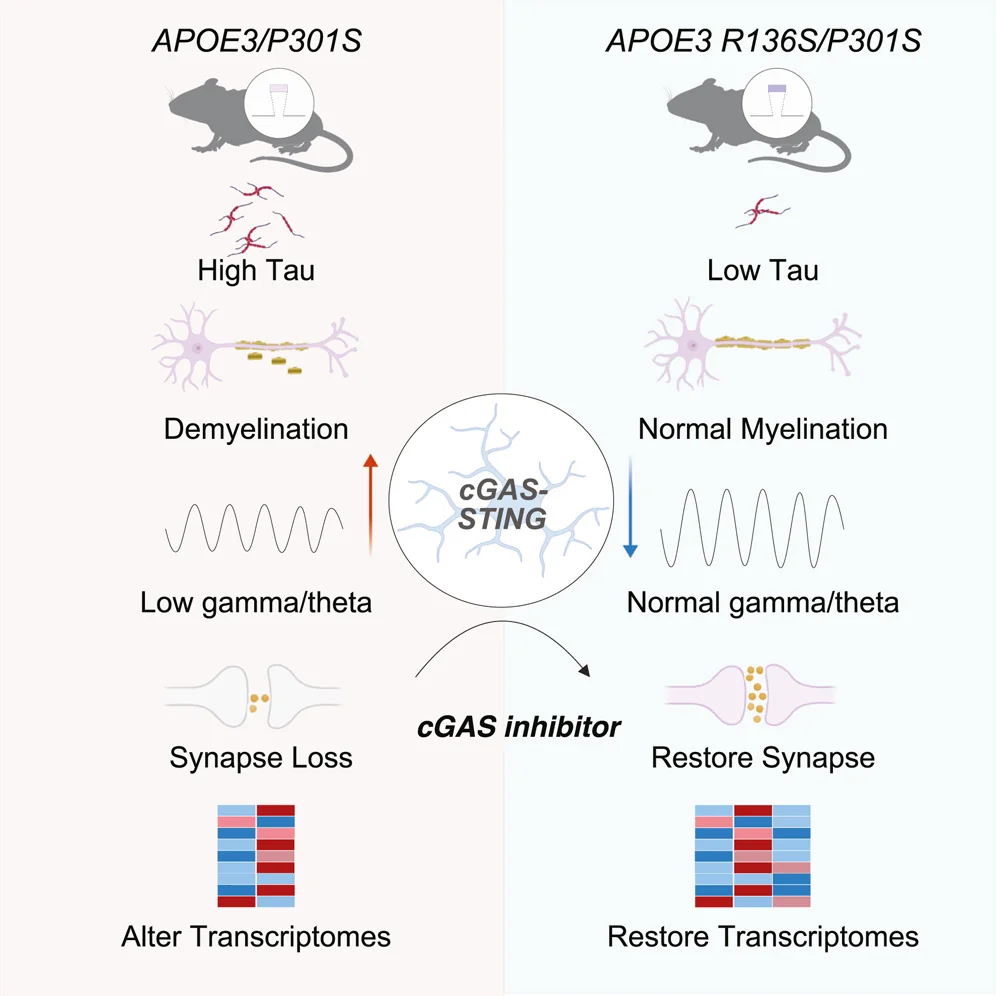
The researchers used CRISPR/Cas9 to create a mouse model In which the native mouse APOE gene was replaced with either the normal human APOE3 gene or the protective APOE3 with the Christchurch mutation. These mice were then crossed with an established mouse model of tauopathy (P301S) to study the mutation’s effects in the presence of tau pathology independently of amyloid plaques. Mutation-carrying mice showed a marked decrease in the accumulation of aggregated tau in the hippocampus. The mutation also protected against the loss of synapses, preserving levels of synaptophysin and the post-synaptic protein PSD95.
One of tauopathy’s most insidious symptoms is myelin loss, which harms neuronal function. Mice with the mutation were protected, demonstrating upregulation of myelin basic protein (MBP) and other myelination markers in the hippocampus.
Functionally, the mutation prevented the tau-induced decline in theta and gamma power in the hippocampus. Theta and gamma waves are rhythms of synchronized electrical activity generated by large groups of neurons. They are fundamental to how the brain processes information, especially for memory. A decline in theta and gamma power indicates that the brain’s circuits are not effectively communicating and coordinating.
This circuit-level disruption is a direct underlying cause of the cognitive impairments seen in Alzheimer’s, such as difficulty forming new memories. The paper notes that these deficits can appear long before neurons die, making them an early indicator of disease.
“We are particularly encouraged that this mutation ameliorates disease at the level of brain function, which has not been shown before,” said Dr. Sarah Naguib, the study’s co-first author.
Interestingly, the researchers did not use the usual physical tests, such as the Morris water maze, to assess cognitive function. Theta and gamma waves may be more direct markers of brain activity than physical manifestations, which can be influenced by many external factors.
Inflammation is the key
The researchers also ran in vitro experiments in mouse microglia, the brain’s resident immune cells, and in human microglia derived from induced pluripotent stem cells. In the brain, APOE genes are mostly expressed in microglia and astrocytes but much less so in neurons.
The experiments showed that microglia with the R136S mutation were more efficient in the uptake and clearance of tau. This could indicate that the mutated cells excel in preventing extracellular tau from entering neurons, where this protein is most dangerous.
A central finding was that the R136S mutation suppressed the cGAS-STING-interferon signaling pathway in microglia. This pathway, a central regulator of inflammation, is normally activated by tau pathology and is known to be a major driver of Alzheimer’s progression and symptoms.
Treating tauopathic mice that had the normal APOE3 gene with a cGAS inhibitor recapitulated many of the mutation’s benefits, reducing tau spread and protecting against synaptic loss. The treatment caused highly correlated gene expression changes in both microglia and excitatory neurons.
“This is an exciting study because it suggests that inhibiting this cGAS-STING pathway could make the brain more resistant to the Alzheimer’s process, even in the face of significant tau accumulation,” said the study’s senior author, Dr. Li Gan, director of the Helen and Robert Appel Alzheimer’s Disease Research Institute at Weill Cornell Medicine. “We can’t engineer the rare Christchurch mutation into people to prevent Alzheimer’s, but targeting the same pathway it modulates could offer a new therapeutic strategy for Alzheimer’s and potentially other neurodegenerative conditions.”
LIterature
[1] Naguib, S., Lopez-Lee, C., Torres, E. R., Lee, S.-I., Zhu, J., Zhu, D., Ye, P., Norman, K., Zhao, M., Wong, M. Y., Ambaw, Y. A., Muñoz-Castañeda, R., Wang, W., Patel, T., Bhagwat, M., Norinsky, R., Mok, S.-A., Walther, T. C., Farese, R. V., … Gan, L. (2025). The R136S mutation in the APOE3 gene confers resilience against tau pathology via inhibition of the cGAS-STING-IFN pathway. Immunity.
[2] Quiroz, Y. T., Aguillon, D., Aguirre-Acevedo, D. C., Vasquez, D., Zuluaga, Y., Baena, A. Y., … & Arboleda-Velasquez, J. F. (2024). APOE3 Christchurch heterozygosity and autosomal dominant Alzheimer’s disease. New England Journal of Medicine, 390(23), 2156-2164.







