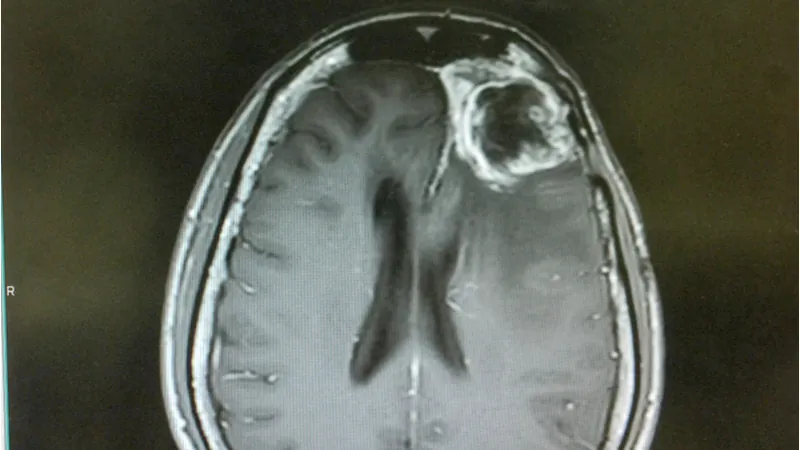
A report in the Journal of Cell Science has outlined different stages of cellular senescence alongside a mechanism that reverses it, along with a potential new treatment for glioblastoma.

Read More
Old paradigms in cellular senescence
Cellular senescence contributes to aging and age-related diseases, likely primarily through the inflammatory SASP. Senescence is often discussed in black-and-white terms: a cell is either senescent or it isn’t. It’s also widely accepted that once a cell enters a senescent state, its cell cycle arrest is permanent. However, more research is coming to light that paints a more complicated picture, including a recent study from the Homi Bhabha National Institute that challenges both of these assertions. [1]
On first consideration, the reversal of senescence may seem like a good thing. However, given the anti-cancer effects of senescence, the greater result of its reversal may be tumorigenesis rather than an anti-aging effect. In fact, previous research by the same lab has presented evidence of various cancer cells becoming senescent after treatment with anti-cancer therapies and then later resuming their proliferation.
Ciprofloxacin induces senescence in glioblastoma cells
Ciprofloxacin is an antibiotic that has also shown anti-tumor properties in some old, largely overlooked studies. However, these studies did not investigate a mechanism of action for these effects. Here, the researchers first showed it inhibited growth in four tumor cell lines in vitro primarily by inducing senescence. Ciprofloxacin caused an increase in SA ß-gal staining, SASP factors, reactive oxygen species (ROS), DNA damage, and senescence gene signatures via RNAseq. Many of these measures became positive in upwards of 80% or 90% of cells.
When ciprofloxacin was removed after 15 days, cells did not begin to proliferate, indicating that their senescence was not reversed. However, cells in which the treatment was removed after 5 or 10 days resumed proliferation. These cells were sorted by size (senescent cells typically show an enlarged morphology) to test whether senescent cells were returning to proliferation or if the proliferation observed was simply from the non-senescent cells within this treated population. The larger cells showed extensive SA ß-gal staining and, after a short delay, proliferated to equal numbers as the smaller, SA ß-gal negative cells.
p65 moves into the cell nucleus during senescence reversal
Whole-transcriptome analysis identified the p65 gene network as highly differential in ciprofloxacin-treated cells. Diving deeper, the researchers found that p65 was localized primarily in the cytoplasm of senescent cells and in the nucleus after removal of the treatment. Preliminary experiments suggested that SMAD7 may mediate this process. However, this did not occur in the cells that were treated for 15 days, which did not resume proliferation.
To test whether movement of p65 to the nucleus was involved in reversing senescence in the 5- or 10-day treated cells, the researchers also treated the cells with drugs that prevented this movement of p65. These cells showed increased levels of senescence, especially with respect to ROS-related measures. Rather than return to proliferation after ciprofloxacin removal, these cells began to die. When implanted into mice, cells treated for 10 days with ciprofloxacin (both the senescent and non-senescent subpopulations) formed tumors while cells treated for 15 days or for 10 days plus a p65 nuclear localization inhibitor did not.
Here, we demonstrate that ciprofloxacin-induced senescence in glioma-derived cell lines and primary glioma cultures is defined by SA-ß-gal positivity, a senescence-associated secretory phenotype (SASP), a giant cell (GC) phenotype, increased levels of reactive oxygen species (ROS), ?-H2AX and a senescence-associated gene expression signature, and has three stages of senescence –initiation, pseudo-senescence and permanent senescence. Ciprofloxacin withdrawal during initiation and pseudo-senescence reinitiated proliferation in vitro and tumor formation in vivo. Importantly, prolonged treatment with ciprofloxacin induced permanent senescence that failed to reverse following ciprofloxacin withdrawal. RNA-seq revealed downregulation of the p65 (RELA) transcription network, as well as incremental expression of SMAD pathway genes from initiation to permanent senescence. Ciprofloxacin withdrawal during initiation and pseudo-senescence, but not permanent senescence, increased the nuclear localization of p65 and escape from ciprofloxacin-induced senescence. By contrast, permanently senescent cells showed loss of nuclear p65 and increased apoptosis. Pharmacological inhibition or genetic knockdown of p65 upheld senescence in vitro and inhibited tumor formation in vivo. Our study demonstrates that levels of nuclear p65 define the window of reversibility of therapy-induced senescence and that permanent senescence can be induced in GBM cells when the use of senotherapeutics is coupled with p65 inhibitors.
Conclusion
The framework presented by this study suggests three stages of senescence: initiation, pseudo-senescence, and permanent senescence. During pseudo-senescence, many of the typical characteristics of cellular senescence can be seen, but this state is reversible. Stopping a senescence-inducing, anti-cancer therapy too early may result in partially senescent cells returning to proliferation. However, this study also opens the door to combination therapies that block this reversal in order to prevent cancer recurrence.
In addition to the study’s relevance to the age-related disease of cancer, it also has important implications for the broader longevity field. More and more, scientists are recognizing the heterogeneity of senescent cells based on original cell type, senescence inducer, and other factors. So too, must they now grapple with the added complication of senescence as a spectrum, rather than a binary switch that is turned on or off.
The question remains, what should ultimately become of these pseudo-senescent cells when designing a longevity treatment? Should they be cleared by senolytics or left alone? Is there a safe way to reverse senescence in these cell populations without triggering cancerous proliferation? Furthermore, what do these cells look like in non-cancerous cell lines? Armed with this knowledge, researchers can build more targeted therapeutics to help minimize side effects and maximize benefits.
Literature
[1] Salunkhe, S., et al. Nuclear localization of p65 reverses therapy-induced senescence. Journal of Cell Science (2021). https://doi.org/10.1242/jcs.253203