Impaired Brain Blood Flow Might Be Important in Alzheimer’s
- A CO2-based diagnostic was far more accurate than an amyloid-based one.
- Under the vascular hypothesis of Alzheimer’s, the disease is primarily characterized by the blood that the brain receives.
- A diagnostic test based on this hypothesis outperformed other, well-established, diagnostics.
Scientists have developed a potent diagnostic tool based on the vascular hypothesis for Alzheimer’s. It outperformed three current techniques and might offer clues to the mechanism behind the disease [1].
The blood flow hypothesis
Traditionally, Alzheimer’s disease has been associated with the presence of amyloid plaques and tau tangles in the brain: the amyloid cascade hypothesis. However, recent studies have engendered doubts about it [2]. For instance, researchers have noted that many people with significant amyloid buildup never develop dementia [3] and that cerebrovascular disease is a common feature of Alzheimer’s [4]. This has led to the vascular hypothesis, which suggests that problems with the brain’s blood supply are a key part of this disease.
In a new study from the University of Southern California, scientists propose that a breakdown in cerebral perfusion dynamics leads to reduced blood flow (hypoperfusion) and hypoxia. Hypoxia can, in turn, increase the production of amyloid, creating a vicious cycle in which vascular damage and amyloid pathology reinforce each other and lead to cognitive decline.
Building on this idea, they set out to create a superior diagnostic tool for Alzheimer’s. Today, early diagnostics involve either a spinal tap or a PET scan to measure amyloid content. “Physicians take the emissions from that PET radioactive tracer as an approximate measure of how much amyloid or tau the person has in their brain,” said Vasilis Marmarelis, Dean’s Professor in the Alfred E. Mann Department of Biomedical Engineering. “Speaking from experience, after having seen data in my own study, I can tell you that it’s very inadequate. But it’s the gold standard, although most physicians don’t do it because it’s very expensive.”
Building the new diagnostic tool
Another popular option involves cognitive assessment tests, such as the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). However, they have their own flaws. “The MoCA does give you an idea about the cognitive abilities of a person,” Marmarelis explained. “But these are not biological or physiological tests. These are just behavioral tests that are subject to all kinds of biases, imperfections and errors.”
The researchers recruited 167 participants and categorized them into three groups: cognitively normal, mild cognitive impairment (MCI), or mild Alzheimer’s disease. Each participant then underwent a non-invasive procedure in which researchers recorded several signals simultaneously. This included using transcranial Doppler, an ultrasound to measure blood flow speed in major brain arteries; near-infrared spectroscopy, which uses light to measure oxygen levels in the prefrontal cortex;, and continuous monitoring of arterial blood pressure and end-tidal CO₂.
“When we exert cognitive effort, we generate CO₂ from the metabolism in our cerebral cells, which obviously has to be taken away by our blood to avoid acidosis,” Marmarelis said. “Our body is endowed with this regulatory mechanism called vasomotor reactivity, which dilates our cerebral vessels when CO₂ goes up in the blood, so that more blood can go through and the excess CO₂ can be washed out.”
However, in Alzheimer’s patients, this regulatory mechanism begins to fail. “They cannot dilate the cerebral vessels to bring more blood in and provide adequate blood perfusion to the brain,” Marmarelis expalins. “This means they don’t get the oxygen, nutrients and glucose that we need for cognition in a timely manner.”
CDI beats the rest
Given the importance of early diagnostics of Alzheimer’s, the team’s goal was to develop and test a novel marker that they called the Cerebrovascular Dynamics Index (CDI). This is a single, composite score that counts how well blood vessels react to changes in CO₂, how well the brain maintains stable blood flow when blood pressure changes, and, finally, how well oxygen levels in the cortex are maintained during changes in both blood pressure and CO₂.
CDI performed extremely well compared to the conventional methods used as positive controls. The study measured performance using “area under the curve” (AUC), a statistical measure in which 1.0 represents a perfect test and 0.5 is no better than chance.
In diagnosing MCI/AD patients vs. healthy controls, CDI achieved an AUC of 0.96. This was much higher than the other physiological test used, the amyloid PET scan, which had an AUC of 0.78. CDI also narrowly outdid the two cognitive tests, MoCA (0.92) and MMSE (0.91).
CDI was also the strongest performer for determining disease severity (differentiating MCI from AD). It achieved AUC of 0.98, higher than MMSE (0.83) and MoCA (0.78). It crushed the amyloid PET scan (0.61). In fact, this last result was not even statistically significant, indicating that amyloid levels alone were not useful for this task.
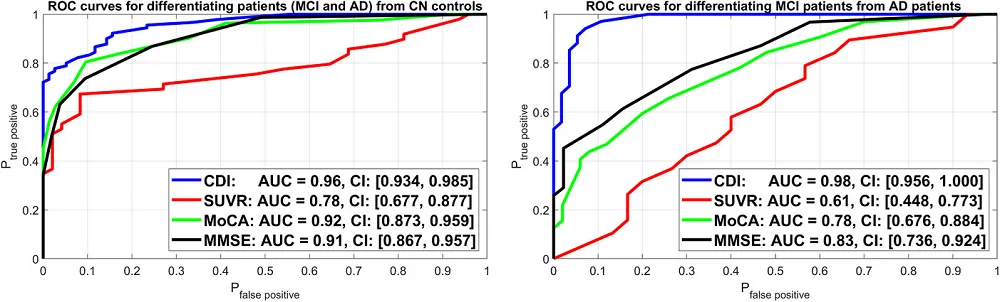
“What we have that others didn’t have before is a methodology to quantify these dynamic relations that’s extremely robust and accurate,” Marmarelis said. “We can now differentiate patients with mild cognitive impairment and Alzheimer’s from cognitively normal controls far better than the PET measurement and even better than the MoCA neurocognitive test.”
However, this study’s importance potentially goes beyond diagnostics. It might help uncover hidden truths about Alzheimer’s and influence the development of therapies. According to Marmarelis, the results indicate “that the particular aspect of dysregulation of cerebral perfusion regulation may be the critical aspect in the pathogenesis of this disease, probably in conjunction with other factors, including amyloid accumulation.”
Literature
[1] Marmarelis, V., Billinger, S., Joe, E., Shin, D., Hashem, S., Rizko, J., … & Chui, H. C. (2025). Dysregulation of cerebral perfusion dynamics is associated with Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 17(3), e70134.
[2] Kepp, K. P., Robakis, N. K., Høilund-Carlsen, P. F., Sensi, S. L., & Vissel, B. (2023). The amyloid cascade hypothesis: an updated critical review. Brain, 146(10), 3969-3990.
[3] Aizenstein, H. J., Nebes, R. D., Saxton, J. A., Price, J. C., Mathis, C. A., Tsopelas, N. D., … & Klunk, W. E. (2008). Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology, 65(11), 1509-1517.
[4] De la Torre, J. C. (1994). Impaired brain microcirculation may trigger Alzheimer’s disease. Neuroscience & Biobehavioral Reviews, 18(3), 397-401.







