Heat May Speed Up Epigenetic Aging in Older Adults
- Results from different epigenetic clocks differ, especially when analyzing short-term time widows.
A recent study reported significant associations between increased heat days and accelerated epigenetic aging [1].
Heat alters DNA
While warm summer days on the beach are pleasant, extreme heat is not as enjoyable, especially in daily life. It is also not healthy, since extreme heat has been linked to cardiovascular diseases and death [2, 3].
DNA methylation is a biological process that is known to respond to stressors, such as heat. By altering how DNA is methylated, organisms can react to stress by modifying their gene expression. These short-term, stress-related changes might have wider, long-term consequences that can eventually impact lifespan.
While there is ample evidence in different organisms, from worms [4] to mice [5], that heat impacts DNA methylation patterns, studies in humans are scarce. Therefore, these researchers used epigenetic clocks, which measure DNA methylation patterns, to assess whether outdoor heat has an effect on the speed of aging. They analyzed data from a representative sample of over 3,500 adults, aged 56 and up, in the United States.
Hot and humid
Those researchers calculated a daily heat index based on a National Weather Service (NWS) formula for each day between 2010 and 2016 in the contiguous United States. The heat index considers the daily maximum ambient temperature and the minimum relative humidity to approximate how the human body feels temperature. It can differ with the same ambient temperature but different humidity; with lower humidity, the apparent temperature can be lower than the air temperature.
“It’s really about the combination of heat and humidity, particularly for older adults, because older adults don’t sweat the same way. We start to lose our ability to have the skin-cooling effect that comes from that evaporation of sweat,” said Jennifer Ailshire, senior author of the study and professor of gerontology and sociology at the USC Leonard Davis School. “If you’re in a high-humidity place, you don’t get as much of that cooling effect. You have to look at your area’s temperature and your humidity to really understand what your risk might be.”
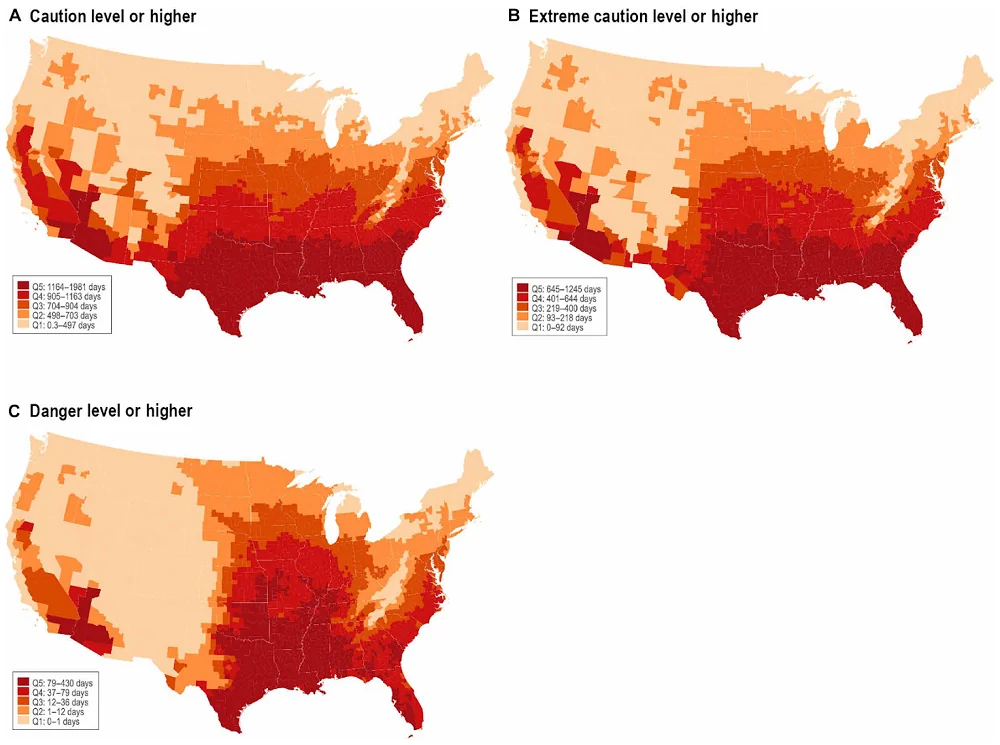
The heat index is an estimate of the stress that heat exerts on the human body and the potential for adverse health effects, and it is divided into categories that reflect it. Values between 80° to 90°F (26.7° to 32.2°C) and labeled “caution” and suggest increasing concern. Values between 90° and 103°F (32.2° and 39.4°C) are labeled “extreme caution” and indicate a moderate health risk, and values between 103° and 124°F (39.4° and 51.1°C) are labeled “danger”, indicating a high risk of adverse health effects. Finally, values above 124°F (51.1°C) are labeled “extreme danger”, as such temperatures are known to cause rapid heatstroke.
The researchers examined different time windows in their analysis. By analyzing shorter time windows, they estimated the effects of immediate heat waves. Mid-length windows allowed them to observe delayed responses to heat exposure, and long-term windows show the consequences of prolonged exposure to heat, including its cumulative health impact and potential dose-response effect.
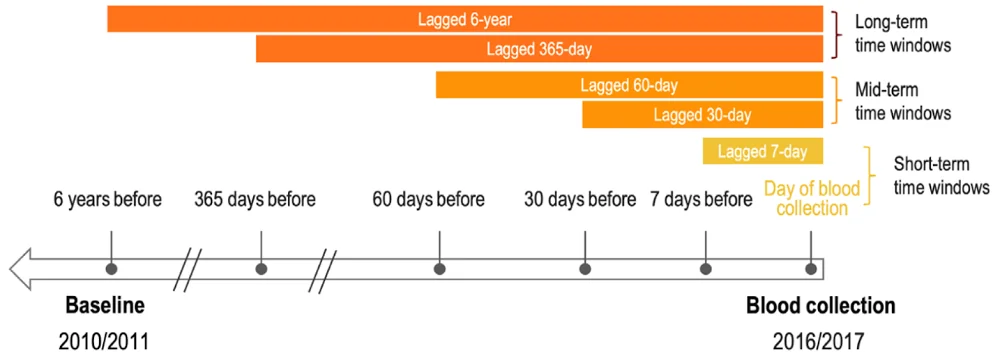
However, the researchers were not able to take multiple methylation measurements at various time points throughout the study. This would have allowed them to track methylation changes in response to heat days.
Hotter days, quicker aging
Based on the available data, the researchers reported “significant associations between heat and accelerated epigenetic aging that differ across epigenetic clocks.”
A co-author of the study, Eunyoung Choi, USC Leonard Davis PhD in Gerontology alumna and postdoctoral scholar, commented on the results: “Participants living in areas where heat days, as defined as Extreme Caution or higher levels (≥90°F), occur half the year, such as Phoenix, Arizona, experienced up to 14 months of additional biological aging compared to those living in areas with fewer than 10 heat days per year,” Choi said. “Even after controlling for several factors, we found this association. Just because you live in an area with more heat days, you’re aging faster biologically.”
The longer, the worse
While the general conclusions were clear, there were differences in the more granular levels depending on the epigenetic clock used.
The PCPhenoAge epigenetic clock showed an association between the number of heat days and accelerated aging, at all levels of heat intensity and in all time windows. PCGrimAge and DunedinPACE also indicated the effect of heat on epigenetic age, but these results were only significant for longer time windows, not for the short and mid-length periods.
These differences might stem from variations in the methylation sites selected to build each clock, the various aspects of aging on which each clock focuses, and the different sensitivities to environmental stresses that each clock represents. If researchers want to understand the differences more deeply, they need to investigate the changes in methylation of specific sites and their short- and long-term impact on biological processes.
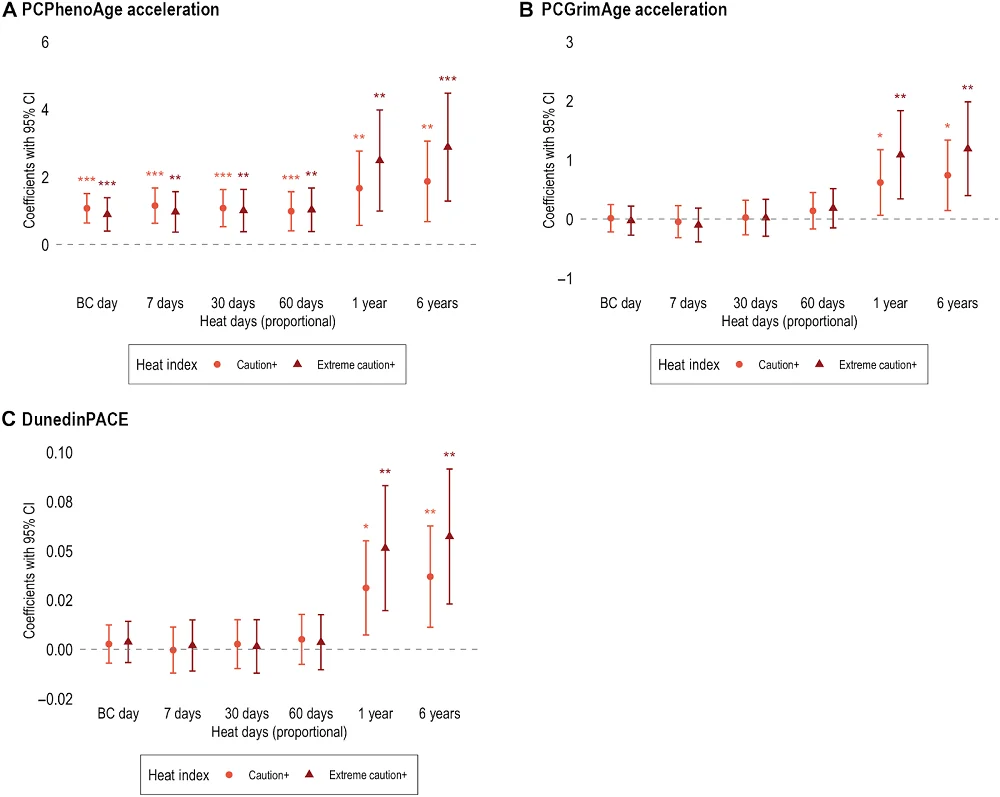
Additionally, the differences in results regarding different time frames might reflect the differences in biological processes activated as a response to short-term compared to long-term heat stress. The effects observed on a short-term scale might be less robust and possibly transient. On the other hand, the long-term effects of heat exposure might accumulate over time. This is supported by previous studies that have also seen more pronounced effects of heat on methylation over longer periods than shorter ones [6, 7].
The authors also point to the possibility that extended periods of heat can change behavior, such as reducing physical activity and leading to increased stress and anxiety, which results from heat-related sleep disruptions and discomfort. Accumulated over time, these factors can result in health decline and accelerated aging.
Everyone is affected
When the researchers analyzed different sociodemographic subgroups based on age, gender, race/ethnicity, education, and wealth, they concluded that there was consistency among the subgroups, and their results did not suggest a higher vulnerability of any specific group. However, the researchers suggest that a lack of a subgroup-specific epigenetic clock might limit this part of the analysis.
This analysis is based on the weather conditions outdoors. However, as researchers admit, they cannot assess how much time the participants spend outdoors and whether they use air-conditioning. Therefore, those results should be interpreted “as reflecting the potential for heat exposure rather than direct, personal heat exposure.”
Risk mitigation
The authors stress that their results show the importance of including hot weather when discussing morbidity and mortality risk factors. Mitigating the risks of excessive heat should be considered when designing public policy and developing public health interventions.
“If everywhere is getting warmer and the population is aging, and these people are vulnerable, then we need to get really a lot smarter about these mitigation strategies,” Ailshire concluded.
Literature
[1] Choi, E. Y., & Ailshire, J. A. (2025). Ambient outdoor heat and accelerated epigenetic aging among older adults in the US. Science advances, 11(9), eadr0616.
[2] Cleland, S. E., Steinhardt, W., Neas, L. M., Jason West, J., & Rappold, A. G. (2023). Urban heat island impacts on heat-related cardiovascular morbidity: A time series analysis of older adults in US metropolitan areas. Environment international, 178, 108005.
[3] Khatana, S. A. M., Werner, R. M., & Groeneveld, P. W. (2022). Association of Extreme Heat With All-Cause Mortality in the Contiguous US, 2008-2017. JAMA network open, 5(5), e2212957.
[4] Wan, Q. L., Meng, X., Dai, W., Luo, Z., Wang, C., Fu, X., Yang, J., Ye, Q., & Zhou, Q. (2021). N6-methyldeoxyadenine and histone methylation mediate transgenerational survival advantages induced by hormetic heat stress. Science advances, 7(1), eabc3026.
[5] Murray, K. O., Brant, J. O., Iwaniec, J. D., Sheikh, L. H., de Carvalho, L., Garcia, C. K., Robinson, G. P., Alzahrani, J. M., Riva, A., Laitano, O., Kladde, M. P., & Clanton, T. L. (2021). Exertional heat stroke leads to concurrent long-term epigenetic memory, immunosuppression and altered heat shock response in female mice. The Journal of physiology, 599(1), 119–141.
[6] Ni, W., Nikolaou, N., Ward-Caviness, C. K., Breitner, S., Wolf, K., Zhang, S., Wilson, R., Waldenberger, M., Peters, A., & Schneider, A. (2023). Associations between medium- and long-term exposure to air temperature and epigenetic age acceleration. Environment international, 178, 108109.
[7] Chiu, K. C., Hsieh, M. S., Huang, Y. T., & Liu, C. Y. (2024). Exposure to ambient temperature and heat index in relation to DNA methylation age: A population-based study in Taiwan. Environment international, 186, 108581.







