Fixing Sugar Metabolism Shows Promise Against Dementia
- This may have effects on a significant component of Alzheimer's.
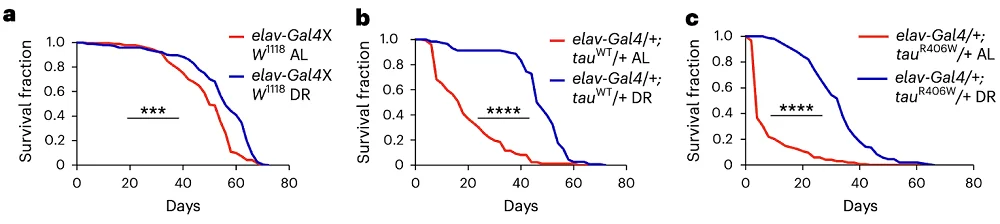
- In flies with tauopathies, dietary restriction helped lifespan significantly.
- The researchers suggest that this is due to an interaction between glycogen and tau.
Scientists have shown that aberrant metabolism of glycogen in neurons is linked to the accumulation of harmful tau protein. Caloric restriction, genetic interventions, and small molecules might help [1].
Glycogen and the brain
Aberrant aggregation of microtubule-associated protein tau (MAPT), or simply tau protein, is a hallmark of several neurodegenerative diseases [2]. The most famous of them is Alzheimer’s disease, in which tau accumulation in the form of hyperphosphorylated neurofibrillary tangles (NFTs) damages neurons.
Another, less-known, characteristic of many of these diseases is abnormal glycogen metabolism and accumulation [3]. Glycogen is a stored form of glucose, used by the body as an energy source when nutrient levels are low. It is mostly found in the liver and muscle, but brain cells (predominantly astrocytes but also neurons) also contain small amounts of it.
Impaired glycogen metabolism in neurons hurts learning and memory, while dietary restriction (DR) is known to extend lifespan and delay neurodegeneration in animal models of neurodegenerative diseases. In this new study published in Nature Metabolism, scientists from the Buck Institute for Research on Aging tried to understand how these two facts might be connected.
Diet restriction rescues lifespan
The authors started with two Drosophila fly models. One exhibited accelerated accumulation of wild-type tau protein, while the other included a known mutation in MAPT (R406W), which, in humans, causes a severe familial disease called frontotemporal lobar degeneration with tau inclusions (FTLD-tau).
The flies were either freely fed or restricted in calories. DR significantly increased lifespan even in healthy controls. In the two disease models, the effect was even more dramatic. DR rescued lifespan in flies with aberrant accumulation of wild-type tau almost completely, and in mutation-carrying flies, the difference was highly significant. Accordingly, in DR flies, levels of neuronal death fell dramatically.
Proteomic analysis of the flies’ brains revealed that pathways related to fat and glycogen metabolism were among the most drastically changed by DR, and glycogen levels were indeed elevated in the brains of tauopathic flies.
Interestingly, however, DR did not seem to alter overall levels of glycogen, despite clearly having a strong beneficial impact. The researchers suspect that what might be important is the rate of glycogen turnover. The enzymes involved in this turnover, including glycogen phosphorylase (GlyP), were upregulated in mutant flies on DR. Overexpression of GlyP increased the lifespan of mutant flies by almost 70% and drastically reduced neuronal death.
More antioxidants!
The researchers used metabolomics and RNA sequencing to study the molecular effects of GlyP upregulation. Surprisingly, the pathways for energy production, namely glycolysis and the citric acid cycle, were actually downregulated. Instead, the glucose from the broken-down glycogen was being shunted into the pentose phosphate pathway (PPP). Its primary function is to generate antioxidants: molecules that combat oxidative stress. Reactive oxygen species (ROS) were indeed significantly reduced in the brains of the flies with enhanced glycogen breakdown.
According to the researchers, this might at least partially explain the benefits of DR and GlyP upregulation. In line with this hypothesis, blocking the PPP with a small molecule abolished the protective effects of glycogen breakdown. The team also successfully recreated the effects of genetic GlyP overexpression by using another small molecule, 8-Bromo-cAMP, to activate the GlyP-producing pathway.
A vicious cycle?
The team then ran experiments in vitro on human neurons derived from induced pluripotent stem cells (iPSCs) that were obtained from patients with FTLD-tau. Genetically corrected cells from the same donors were used as controls. The researchers demonstrated increased glycogen accumulation in FTLD-tau cells and also tested the rescue mechanism by overexpressing the human version of the glycogen breakdown enzyme (PYGB) in the diseased human neurons. This reduced abnormal glycogen accumulation and restored mitochondrial abundance, which declines with this disease.
Importantly, using these human neurons, the team showed that tau protein and glycogen co-localize within cells and physically interact, supporting the hypothesis that a direct interaction between the two might be part of the problem. The authors hypothesize that this may create a detrimental vicious cycle in which tau binding promotes glycogen accumulation, which, in turn, exacerbates tau pathology and oxidative stress.
“Our findings suggest that glycogen is more than just a metabolic reservoir – it may act as a sticky trap for tau, creating a dangerous feedback loop where tau promotes glycogen buildup, and glycogen in turn fuels tau aggregation,” said Dr. Pankaj Kapahi, the corresponding author of the study, to Lifespan.io. “Breaking this cycle could open a new therapeutic front in the fight against Alzheimer’s disease.”
Literature
[1] Bar, S., Wilson, K. A., Hilsabeck, T. A., Alderfer, S., Dammer, E. B., Burton, J. B., … & Kapahi, P. (2025). Neuronal glycogen breakdown mitigates tauopathy via pentose-phosphate-pathway-mediated oxidative stress reduction. Nature Metabolism, 1-17.
[2] Goedert, M., Eisenberg, D. S., & Crowther, R. A. (2017). Propagation of tau aggregates and neurodegeneration. Annual review of neuroscience, 40(1), 189-210.
[3] Mann, D. M. A., Sumpter, P. Q., Davies, C. A., & Yates, P. O. (1987). Glycogen accumulations in the cerebral cortex in Alzheimer’s disease. Acta neuropathologica, 73, 181-184.








