Among the hallmarks of aging, DNA damage is both one of the most important and one of the hardest to crack. A couple of years ago, when I first learned about Matter Bioworks at a prominent longevity conference, I was amazed at the audacity of the small startup’s vision: actually fixing our DNA, including the mutation burden that accumulates with age.
Behind this vision, however, stands some serious science, and now, Matter Bio has matured into a company with a pipeline and a cash flow. I talked to Sam Sharifi, PhD, Matter Bio’s Chief Scientific Officer, about the company’s philosophy, technology, and research programs, and I caught a glimpse of the future.
Of all things in life – how did you end up founding a longevity biotech company with a bold vision?
I got into longevity very early on. I started studying biology and already wanted to go in the direction of regenerative medicine. Then I came across João Pedro de Magalhães’s website, which was explaining everything. During my master’s, I reached out to him and wanted to do an internship, and that’s how I shifted my focus to longevity.
During my master’s, I went to the European Research Institute for the Biology of Ageing (ERIBA) in Groningen and worked on telomeres in yeast. Then I looked for PhD positions in aging and ended up at the Leibniz Institute on Aging in Jena, where I worked on C. elegans and studied ribosomal RNA genes.
From there, I kept working as a postdoc, but for me, the problem with all this academic work was that they didn’t want to develop therapeutics for aging. Most of the research was fundamental, and if they found something, they would often not pursue developing it further.
At that time, the longevity biotech startup field was just starting to ramp up. I met with a couple of companies and then joined Vincere Biosciences, which makes mitophagy enhancers. During my time at Vincere, I also began working on ideation for what would become Matter Bio.
During this time, On Deck Longevity Biotech (ODLB) came around, and it was perfect timing for me because I was in this transition stage. It was at ODLB that I ended up meeting my co-founder, Chris, since we both had a passion for a similar thesis around aging and DNA damage. There, we worked a bit together to form an idea for a company. Around that time, it was relatively easy to get funding for startups in longevity biotech. Once we got funding, we switched out, and that’s how we founded the company.
Since we had an idea for a DNA editor, we approached George Church, and he said he could help with that, so that’s how he joined as a co-founder. Later on, Jan Vijg and Alex Maslov, who had a sequencing asset, suggested that it might be helpful for our DNA repair and somatic mutations program. They joined as co-founders, and we started acquiring assets from different PIs to help with the spin-off. That’s how the whole of Matter Bio with the different assets was formed in the end.
Like I said, your vision is bold, some would say audacious, but it seems that, like many startups, you went for low-hanging fruits with your current programs (not that there’s anything wrong with that). So, walk me through it: both the vision and the philosophy of what you eventually want to achieve, and what you are doing at this particular stage.
One of the drivers of aging is the accumulation of damage in the genome. Your DNA is a pretty unstable molecule even inside the cell, and it’s the repair mechanisms that try to keep it together. The problem is that with time, you cannot keep up, so the damage starts accumulating. We want to enhance this protection so that you don’t accumulate the damage anymore.
Moreover, if you look at nature and long-lived animals, most of them have very good genome maintenance and DNA repair. We think that this plays a really big role in their longevity. Of course, you have the whole epigenetic reprogramming side, but we wanted to do something that was not addressed yet, and DNA damage seems to be upstream of epigenetic drift as well. There are now more companies coming slowly, but at that time, we were the only ones working on DNA repair because it’s a very challenging and complex thing.
But we thought, “Okay, we are going to look at the long-lived animals and learn from them. What do they do? How do they do it?” And centenarians as well. How do centenarians do it so that we can also do it in “normal” humans?
DNA damage is obviously one of the most important hallmarks of aging, but people were really hesitant to address it because it was so hard. This is why your talk a couple of years ago at a conference, from which I first learned about Matter Bio, resonated with me so much. So, how are things going now?
We started looking at how evolution has dealt with this problem. How does the bowhead whale live to 200 years, the Greenland shark to more than 400, and the naked mole rat to at least 30? We looked at all the long-lived animal genes and started screening for which ones protect human cells against various DNA damage – UV, double-strand breaks, and oxidative lesions.
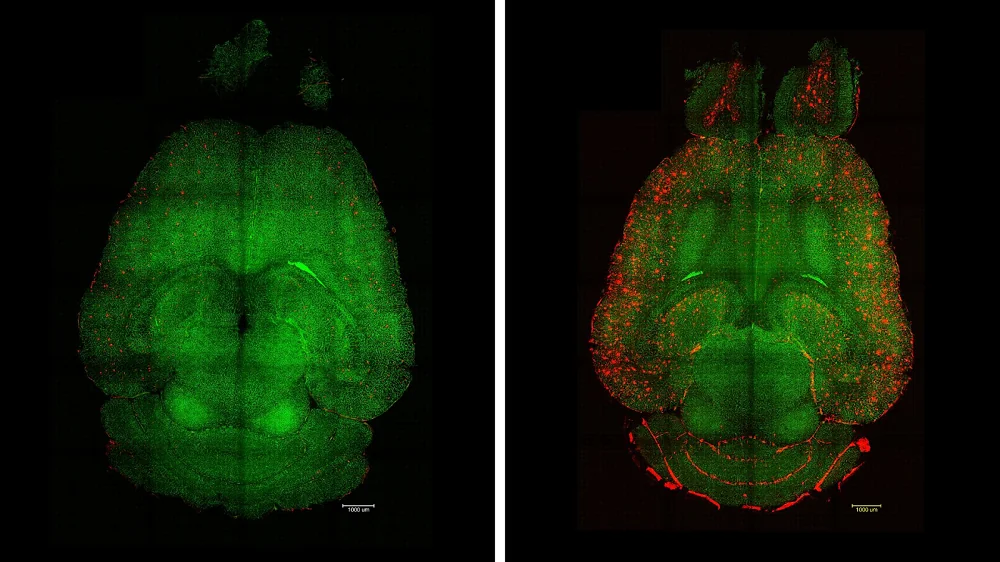
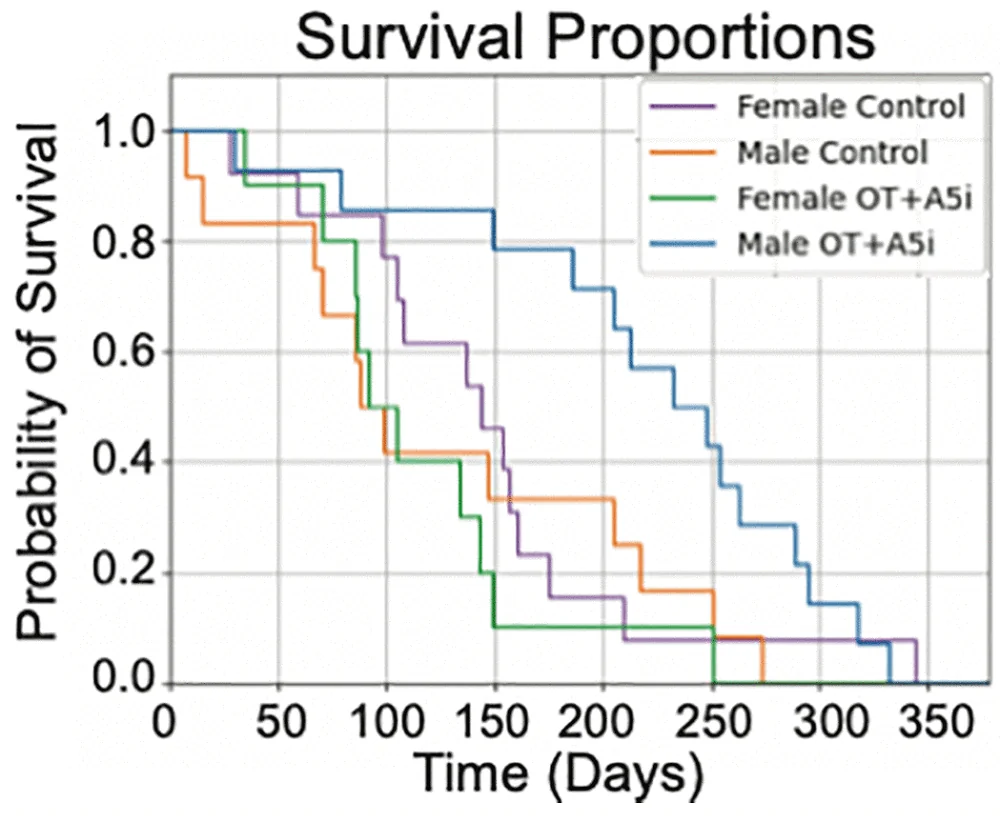
From that, we started making combinations. We found some gene combinations that were interesting, and now we have a couple of combinations that we think are actually protecting the cells. Some of them are very strong, showing an 80% reduction in damage. Now, we want to move that to the in vivo stage and try to enhance DNA repair in mice.
In addition to enhancing DNA repair, you also have this bold vision of fixing existing, long-term damage with this editor that can insert up to 170 kilobases.
Yes, we created a transposon-based editor. Transposons are very good at inserting genetic material, but they’re not targeted, and that has always been a problem. We have manipulated the system to specifically target certain regions directly, but it’s a challenge. We’re still working on that, trying to reduce the off-target rate to a certain level that’s good for healthy people.
With this transposon-based system, we can insert large fragments. Of course, bringing big pieces of DNA into the cell is still a challenge, but we have tested very big fragments, and it’s working.
The idea is, like you said, to replace certain regions that are easily mutated. Some diseases are associated with a gene where mutations are random for each patient. CRISPR cannot really fix that; you have to replace that whole fragment.
We want to start by replacing the mutated parts but also to eventually give better, enhanced versions. Imagine getting the centenarian version of a certain gene, instead of just the normal version that you usually have.
So, you’re not really planning to bring the entire genome back to a youthful state, rolling back most accumulated mutations. You would rather want to preserve function by fixing certain crucial genes, correct? This also means that in the future, such procedures might have to be done regularly – fixing one gene at a time, sort of, “Okay Google, schedule my next DNA fix for next week.”
Exactly. Of course, the genome will still get mutated again, so maybe you’ll have to come in for a fix every couple of years or so. But, yes, we want to focus on certain things, because replacing the whole genome is too much and mutations still occur relatively rarely: roughly 1 per 1 million base pairs.
We want to focus on DNA repair genes, on oncogenes – the critical infrastructure. And what we want to do (which is why we have the sequencing asset) is you would come and have your somatic mutation load checked and then see if you need the treatment or not. We want to have everything in-house: we check your genome for somatic mutations, and then we have the editor to replace them if you need it.
I see a lot of opportunities here: you can protect the genome’s integrity; you can go beyond that and enhance it with beneficial variants. But your tool can also be used to simply duplicate genes, right? Like elephants have several copies of TP53, which helps them suppress tumors.
Yes, that’s part of it. We are also working on that because, like you said, we’ve seen that in elephants, as well as in bowhead whales and some bats, certain genes have multiple copies. For these genes, we are testing if we can give them to humans in multiple copies without getting any side effects. We’re starting in mice, of course, because sometimes these genes are regulated a bit differently, which could be problematic.
Another thing is in vitro versus in vivo. I think your tech might be very good for enhancing autologous stem cells, including iPSCs. It could be complementary to cellular reprogramming, which doesn’t fix the genome, for an even fuller rejuvenation. Do you have something like this in mind?
Yes, we have thought about it. Editing iPSCs is a very good stage to start, especially because if you take the cells from a human at a certain age, their genome will be mutated. You can keep doing epigenetic reprogramming, but at some point, the underlying genome sequence will get too corrupt. I think even if epigenetic reprogramming works very well, at some point, you will have to fix the genome as well.
Currently, we are doing more iMSC editing (iPSC-derived MSCs). We want to start there so that we can start working on iPSCs later on.
The idea is to create more senescence-resistant MSCs that last longer for a therapy. This would be to go more to the translational side – how can we quickly translate such a DNA repair-enhanced therapy for normal humans? I think MSCs are pretty well-established now. If you can make them more senescence-resistant, they can last longer and the therapy will be more effective.
Your most advanced program, though, is something else. Is it nearing Phase 1, correct?
Yes, it’s a therapy using the bacterium Listeria. Here also, we are leveraging millions of years of evolution and co-opting it for treatments. Listeria has evolved to be very good at hiding from the immune system, so we modified the Listeria to leverage that ability to specifically infect the immunosuppressed tumor tissue only. We can then bring cargo to the tumor. We’ve attenuated it such that if it tries to infect other cells outside the tumor, it will be immediately cleared by the immune system.
Our synthetic Listeria goes to the cancer cell, infects it, and then expresses tetanus antigens. These antigens are recognized by the immune system because of your childhood vaccination, and then your immune memory kicks in and clears the cancer cells out. What’s extra exciting is that this works not only very well in the main tumor, but also on metastases, which is critical if we are to treat late-stage disease, which is unfortunately when many patients become symptomatic. We are going to start our first-in-human Phase 1 in Q1 2026.
How does your DNA-fixing technology contribute to this particular program?
We really see it as a continuum: damage accumulates to cause cancer and aging. We want to protect the genome to prevent these diseases, when possible, but we want to cover the other end of the spectrum as well. If you are older and already have accumulated damage, we need an answer to cancer. Those cells don’t benefit from better repair (in fact, the opposite), so clearing them out is critical. So, we want to get rid of cancer cells. It’s pointless to try to replace or protect cells that are too corrupt. If it’s too corrupt, just remove it, that’s the idea.
Using bacteria as a delivery system is an interesting idea, I get it. But you’re basically a DNA-fixing company, so how does this particular program fit into your company’s DNA (pun intended?)
Yes, it’s a bit different from the other assets, but we wanted to be able to intervene at every stage. We have an asset to replace parts of the genome and an asset to repair the genome, but we didn’t have something to remove the cells once they are too damaged. We want to have everything so Matter Bio becomes a full-stack solution. A one-stop shop for your genome in the end.
That makes sense. And I guess this idea was just closer to fruition.
Yes. It was developed in Claudia Gravekamp’s lab at the Albert Einstein College of Medicine, and it was already pretty mature and seemed to be working very well. I was amazed when I saw the data, so we thought we could bring it to the clinic.
We’re now very close to starting to give it to cancer patients beginning next year. The first indication will be pancreatic cancer, which is, of course, very deadly. If we succeed here, it would be a great sign that our method is effective.
You also have something interesting going on with a consortium for analyzing biodata from centenarians and long-lived species. Can you tell me more about it?
We want to collect the biggest biobank of data on long-lived animals and centenarians, specifically. We are planning to get around 1,500 samples from whole-genome sequencing of centenarians.
We hope to find more variants that help protect the genome. One that is known is the centenarian SIRT6 variant, which was found by the labs of Vera Gorbunova, Andrei Seluanov, and Yousin Suh, but there must be more out there. We want to learn more about these and also from long-lived animals. The genomes of many long-lived animals are very poorly studied or annotated.
Recently, some data on the Greenland shark genome came out, but it lacks other complementary -omics data. The rockfish genome is also poorly annotated, and they live 200 years. In the end, what we want to do is multi-omics analysis of these animal and human tissues to help us generate more targets, and by targets, I mean gene variants and other gene solutions like duplication.
You’re contributing your sequencing know-how to this effort, right?
Yes, we will do whole-genome sequencing. At the moment, all of the work on centenarians is whole-exome. There’s no whole genome data, which blew us away. This is a massive opportunity. Of course, with whole-exome, we can get a lot of answers, but we don’t learn a lot about the regulatory regions, which play a crucial role and make up the majority of the genome. The exome is just a fraction of the whole genome (1.5%).
You also have a proprietary sequencing technology that you actually market.
Yes, this came from Jan Vijg’s lab. It’s a form of error-corrected sequencing that helps find specific mutations in the genome. They’re very hard to find with normal sequencing because, normally, you cannot distinguish between sequencing errors and actual mutations.
Our technology is an error-corrected version of next-generation sequencing, called SMM-Seq, where we can actually detect single mutations in a single DNA fragment. We use it internally for our assays, and we also offer it commercially because we saw that other people want to use it. It’s pretty good for a biotech to also sell stuff.
You mentioned the information theory of aging, which is interesting because it’s something David Sinclair talks about, although he focuses on the loss of epigenetic information as the upstream cause of aging. I was wondering, how upstream do you think DNA damage is, and how much can we achieve by fixing just the DNA?
This is a very hard question. I think the epigenome and the genome go hand-in-hand because a lot of mutations will cause a drift in the epigenome. This was recently shown in a paper on the somatic mutation clock, which showed there was a lot of change at methylation sites around where mutations happen.
Of course, that’s not the only thing contributing to aging, but DNA damage appears to be a central regulator: a lot of the other hallmarks of aging are influenced if you cause more DNA damage – senescence, for example. So, I believe that DNA damage is one of, if not the most, upstream of the hallmarks and is the driver of information loss, both genetic and epigenetic, either directly or by inducing reactivity in the cell.
DNA mutation burden actually correlates well with the average lifespan across species, right?
Yes, Alex Cagan’s work is one of the first that came out to show this correlation. Of course, that doesn’t mean causation, but it still suggests that mutations are important and are correlated with the processes that do cause aging.
Before that, people would say, “Mutations don’t really have an effect; the majority are in places where there’s nothing.” For example, there was a study where people knocked out the proofreading polymerase and saw that nothing really happened except that mice were getting cancer, but we don’t know if something else happens because the majority of the animals die of cancer before that. We can’t talk about the effect of DNA mutations on lifespan until we deal with the cancer part.
There also seems to be a maximum number of tolerated mutations per cell, as shown by Alex Cagan’s work, where no matter if you’re a giraffe, a human, or a mouse, your cells don’t seem to be able to sustain more than about 5,000 mutations. So, no matter if it’s because of what’s causing the mutations or the mutations themselves, there’s clearly an upper limit we’re seeing, and it’s not high.
If we look 30 or 40 years into the future, how do you see the anti-aging ecosystem? What would people be doing to slow or reverse aging?
That’s a very interesting futuristic question. In terms of longevity biotech, there will probably be new companies popping up, but also the winners, some really big ones that have already achieved lifespan extension.
I don’t know if we are going to achieve longevity escape velocity any time soon, but there will be companies that make us live longer. I think we’ll see more and more longevity clinics offering therapeutics, and this will be everywhere. I can also see insurance companies being involved because insurers are happy if you don’t get sick, so they would be covering certain longevity therapies as well.
I think that, unlike now when you go to the doctor only when you get sick, and all the other time you just eat well and exercise, it’s going to be continuing action where you get a different procedure once a month or maybe once a week because you’ll have to do a thousand things to stay young.
Yes, I see something like that, too. The question is how far we will get in 30 years. It seems very far on the one hand, but on the other hand, the development of drugs is so slow. Let’s say it’s 10 years to market, so we only have three cycles. It’s not that many anymore, right?
I agree. More in silico, AI-powered studies should help. Does your company use AI?
We are exploring using AI to help us discover new gene combinations based on our screening data. Furthermore, we will use AI to analyze the cross-species multi-omics data. There will be so much data that a normal human wouldn’t be able to process it; you would miss certain things, especially correlations between genes, proteins, and RNA-seq data across different species. AI is much better at looking at multiple layers than a human, and it would help us find targets we would never be able to discover otherwise.
We would like to ask you a small favor. We are a non-profit foundation, and unlike some other organizations, we have no shareholders and no products to sell you. All our news and educational content is free for everyone to read, but it does mean that we rely on the help of people like you. Every contribution, no matter if it’s big or small, supports independent journalism and sustains our future.