In recent years, female reproductive aging has gained much attention due to the increase in women who delay childbearing beyond the age of 35 [1]. At that age, female fertility already starts to decline, and getting pregnant is difficult for many women and carries many risks.
Stages
Female reproductive aging begins much earlier than at menopause. During the Stages of Reproductive Aging Workshop (STRAW), the researchers defined reproductive aging as “a natural process that begins at birth and proceeds as a continuum.” They emphasized that “clearly it is a process and not an event” and created a staging system that can be applied to healthy girls and women. This system relies on biomarkers such as the duration of menstrual cycles and follicle-stimulating hormone (FSH) levels [2].

The authors of that paper considered the increase in FSH levels as “the first measurable sign of reproductive aging.” However, measuring FSH should only be done in the context of estradiol, as elevated estradiol can suppress FSH elevation. Additionally, FSH levels increase gradually and vary greatly when women go through menopausal transition, contributing to difficulties in identifying a cut-off level for stages −3 to +1.
Those researchers also do not recommend using other female hormones to define the stages of female reproductive aging, as their levels vary greatly. Even though they are not best used as indicators of the progression of reproductive aging, the changes in the levels of reproductive hormones are still significant. During the menopausal transition, luteinizing hormone (LH) increases after the change in FSH levels, and estradiol and progesterone levels decrease.
While this staging system provides a uniform nomenclature and reference point, the authors don’t recommend it in circumstances when the female is smoking cigarettes, has a body mass index of under 18 or over 30 kg/m2, exercises heavily (over 10 h/week of aerobic exercise), or has chronic menstrual cycle irregularity, prior hysterectomy, abnormal uterine anatomy such as fibroids, or abnormal ovarian anatomy such as endometrioma.
The authors also note that even healthy women might experience exceptions from this pattern and may go back and forth between stages or skip a stage. This suggests that a lot of unexplored biology behind female reproductive aging awaits further research [2].
Female reproductive span
Fertility is defined as the best estimate of the effect of maternal age on the ability to conceive. It can be calculated in a population that does not use contraceptive methods. A paper by Menkel and collaborators looked at this question in populations living between the 17th and 20th centuries. Their results show that “the rate of childbearing in a population” (fertility) remains relatively stable for women until 30, resulting in more than 400 pregnancies per 1,000 women per year. Past the age of 30, they observed a decline. For women ages 45 or over, fertility rates drop to 100 pregnancies per 1,000 women [3].
Different studies suggest a similar decrease in reproductive capacity with age. First comes a decrease in reproductive capacity (fecundity), which starts to decline around 30 or 35 years of age [1]. Then, women experience the end of their fertility, defined as age at the last birth (for populations that don’t practice modern reproductive methods). The end of female fertility takes place around ten years before menopause and is very variable [4].
In developed nations, menopause usually occurs in women between 45 and 55 [5]. Menopause can be defined retrospectively as a point in time when a woman did not have menstrual cycles for 12 months after her final menstrual period [2].
The timing of natural menopause, as opposed to surgically included menopause, is variable and determined primarily by genetic influences [5]; however, environmental factors also play a role. For example, exposure to environmental endocrine-disrupting chemicals is associated with early menopause [6].
Advances in contemporary medicine have allowed the prolongation of women’s lives by around 30 years compared to the previous century. However, the extension of the female reproductive span lags behind, creating a discrepancy between reproductive span and overall lifespan [7].
Women who are lucky enough to experience longer reproductive spans are also more likely to live longer lives. A few studies investigated the relationship between female reproductive aging trajectories and lifespan. For example, the Women’s Health Initiative observed an association between longer reproductive lifespan or later age at menopause and increased longevity [8]. Another study’s results suggested that a woman who has “the ability to have children late has higher odds of living to exceptionally old age” [9]. Those observations underscore the importance of extending the female reproductive span, not only to have the ability to have children later but also to increase lifespan.
Ovarian reserve: use it or lose it
Female reproductive aging and reproductive span are tightly connected to ovarian reserve and the ovarian follicles. The ovarian follicle is a cellular structure that releases an egg that can be fertilized. Each female has a set number of follicles at birth, and as time passes, those reserves are depleted [10].
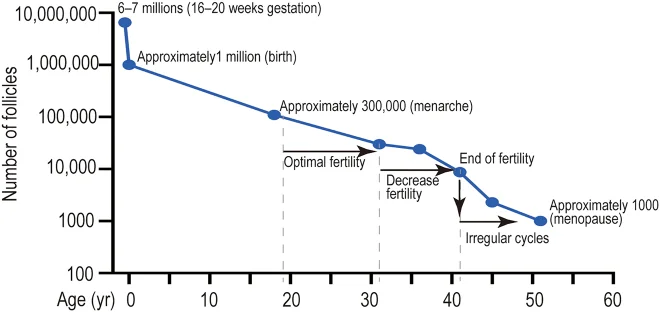
Since there is a wide range in which women experience menopause (42-58 years), it suggests that either the number of follicles at birth varies highly between women or they lose them at different rates [2].
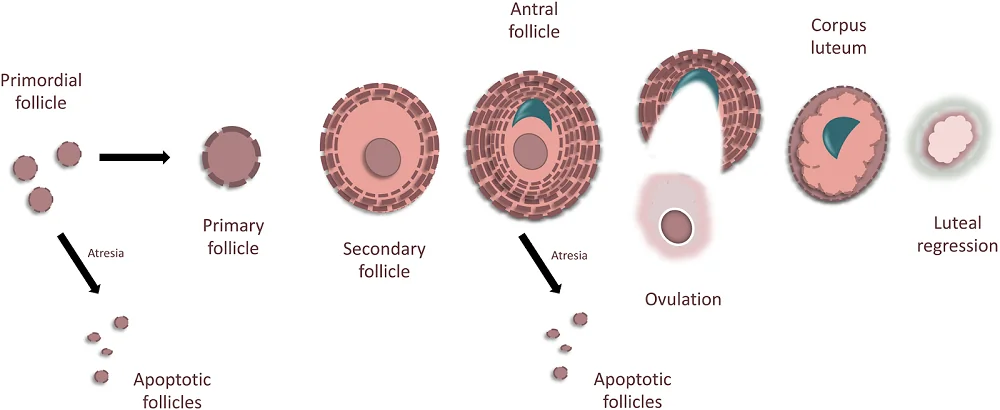
The follicles go through the development process that starts when a female is in her mother’s womb. The starting point for the development of ovarian reserve is primordial follicles.
The oocytes in the primordial follicles need to go through the process of meiosis, which is a cell division that produces reproductive cells (eggs). When oocytes are in the primordial follicles, they start the meiotic process but become arrested at the beginning stage, prophase I. This arrest lasts until the onset of puberty.
Each follicle can embark on one of the three pathways: undergo atresia (an apoptotic cell death), remain quiescent, or become recruited to grow. The first path, atresia, is the most common, and approximately 5 million follicles undergo atresia before a female is even born. The remaining ~2,000,000 remain dormant until they are recruited [11].
When a primordial follicle is recruited, it becomes a secondary follicle and then an antral follicle. With the first menstrual cycle, this antral follicle will either become a dominant follicle, whose role would be to ovulate, or non-dominant, which will become atretic, and its role would be to nourish the dominant follicle. Once a follicle releases an egg, it becomes part of the corpus luteum [12] and undergoes luteal regression if the egg is not fertilized.
This process is controlled by changing hormone levels (estrogen, progesterone, follicle-stimulating hormone, and luteinizing hormone) and is repeated each menstrual cycle. When a woman reaches menopause, she has only about 1000 primordial follicles. The vast majority of follicles and oocytes are lost due to atresia, and it is suggested that atresia might be “a protective mechanism to eliminate poor-quality oocytes that cannot be fertilized” [13, 14].
Research in mice suggests the existence of proliferative germ cells that have the capacity to produce more oocytes and follicles, increasing the ovarian reserve [15]. However, this concept is still debated in the scientific community. Even if such stem cells contribute to increasing the pool of follicles and oocytes, this contribution doesn’t seem relevant enough to impact the onset of menopause [16].
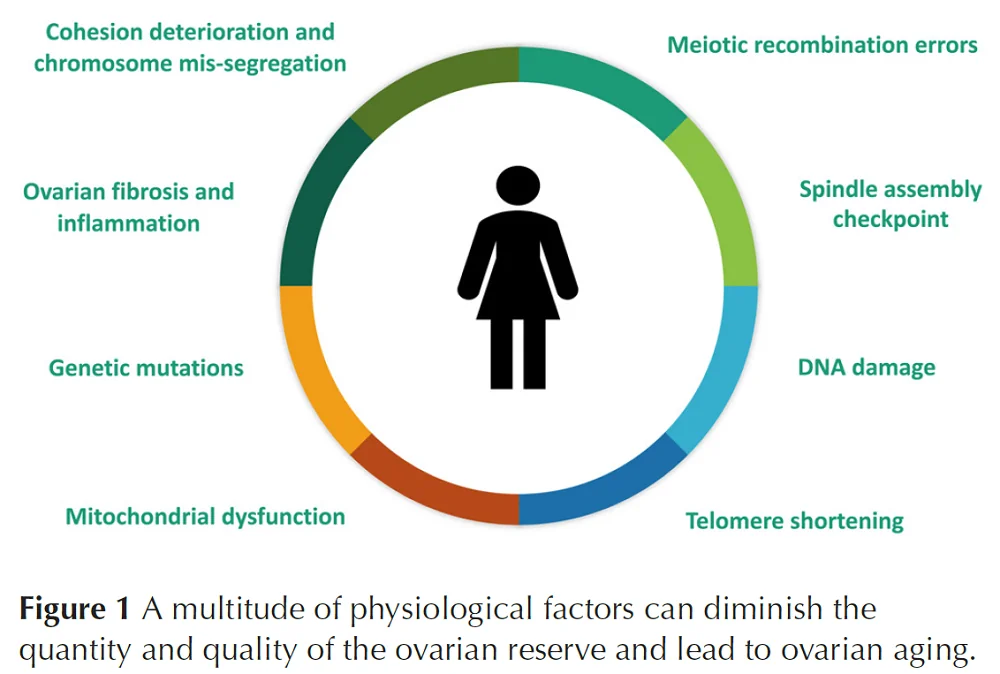
Not only is the quantity of the follicles reduced with age, the quality also suffers. When the activation, development, selection of dominant follicle, and follicle maturation (folliculogenesis) processes become dysregulated due to aging, it leads to poor oocyte quality.
The quality and quantity of ovarian reserve are impacted by multiple factors. The impact of these factors varies among females [13].
Artificial reproductive technologies
Women planning to have children later often rely on artificial reproductive technologies (ART), such as in vitro fertilization (IVF), assuming that these technologies will allow them to have children despite their advanced ages [17]. Unfortunately, research shows that the success rate of ART decreases with age [1].
Two studies investigating the outcomes of in vitro fertilization using donor eggs reported the negative impact of age on IVF outcomes. They observed that the rates of pregnancy and implantations are reduced in the late forties, with the rates of miscarriage increasing [18, 19]. There are also risks to mother and child that increase with age.
Additionally, the long-term effects of ovarian hyperstimulation needed to obtain material for IVF are not well understood. Animal studies have shown “that repeated cycles of ovarian hyperstimulation can accelerate ovarian aging.” [20] Given the number of women who undergo the procedure, the long-term effects of ovarian hyperstimulation on female reproductive aging should be investigated.
Pregnancy risks for older mothers
While the chances of getting pregnant decrease for females starting around mid-30s, those who do get pregnant face the issue of increased health risks associated with pregnancy.
One of the conditions which incidence increases with age is ectopic pregnancy. An ectopic pregnancy occurs when the implantation of a fertilized egg occurs outside the uterus. One of the studies on this topic, based on the French national registry, reports that for patients aged 20–34, the rate of hospital admissions due to ectopic pregnancy was 1.5%. For 35- to 44-year-olds, it increased to 2.5% [1, 21].
Pregnancies in women who are 35 and older are also associated with a higher risk of health complications. The scientific literature lists health problems such as high blood pressure [22, 23], gestational and clinical diabetes [24, 25], and postpartum depression [26]. There is also an increased risk of complications during labor, such as uterine dysfunction [27], and an increased risk of operative delivery, cesarean section, or assisted vaginal delivery [22, 26, 27].
One of the reviews discussing pregnancy outcomes in older women points out that most of the research in this area didn’t address differences in maternal health and lifestyle choices. The authors believe that there is a need to address the risk of delaying pregnancy among healthy women, as those risks might be different (possibly lower) than for those in the general population [26].
Risks to the child
Women are not the only ones that are at risk. Unfortunately, an increased maternal age is also associated with risks for her baby, including stillbirth and unexplained fetal death [28, 29].
The risk of preterm delivery and miscarriage is also increased. Preterm delivery was studied in different populations and countries with similar results [1]. For example, a large population-based study in France from 1995 showed the risk for preterm delivery of 4.5% for women aged 30-34, 5.6% for women aged 35-39, and 6.8% for women 40 or over [30]. A similar study in Canada looking at 160,000 deliveries of singleton newborns reported a risk of prematurity of about 5% in women between 25-34, 6.2% for women between 35-39, and 7.2% for women 40 or over [31].
Most of the studies looking into preterm delivery risk adjusted their analysis from preexisting maternal diseases and other pregnancy complications, which is essential since those exist at higher rates in older women, still showing the increase in risk.
Miscarriage is also associated with age. An analysis performed on over 2,000 women showed that the mother’s age (whether below 25 or above 35) and heavy bleeding were significant risk factors for miscarriage [32].
Probably the most well-known risk of having children late is the increase in the risk of chromosomal defects: aneuploidies. Aneuploidies are abnormalities in the number of chromosomes, such as trisomy 21 (Down syndrome), and the mother’s age is a known risk factor for aneuploidies [33, 34].
On a good note, researchers haven’t found an association between maternal age and most single-gene anomalies. However, since those are rare, there might not be enough data for proper analysis [35].
Babies born to mothers with more advanced age are also at risk for intrauterine growth restriction, a condition that occurs when growth in the womb is slower than expected. Some researchers suggest it is due to placental insufficiency. However, studies have yet to confirm that [1].
A more frequent occurrence in women who delayed childbearing to a later age is multiple pregnancies (e.g., twins). While part of this is a result of assisted reproductive technologies, there is also a clear age-specific effect in spontaneous multiple pregnancies [36, 37]. Researchers analyzing data from France in 1970 (when such technologies were not widely available) showed that multiple pregnancy occurrences were 5.4 per 1,000 in women below 20 years and 14.3 per 1,000 in women between 35 and 39 [1]. Such multiple pregnancies are responsible for some of the adverse perinatal outcomes [38].
Genetics, lifestyle, and environment
A study into genetic factors influencing sge at natural menopause (ANM) investigated the data from ~200,000 women of European ancestry. This study’s researchers identified 290 DNA locations that impact ovarian aging. Further analysis of those genomic locations and experimentation revealed the impact of DNA damage response (DDR) processes on ovarian reserve throughout these women’s lifespan. The results were not specific to only European women but broadly replicated during an analysis of almost 80,000 women of East Asian ancestry, with differences in effect size and frequencies of genetic variants [39].
Childbirth impacts the timing of menopause, with multiple studies showing an association between never delivering a baby (nulliparity) and earlier menopause [40-42]. Another study of over 100,000 women indicated that a higher number of children is associated with a lower risk of early menopause. The same study also concluded that breastfeeding is associated with a lower risk of early menopause [43].
Unsurprisingly, smoking has a negative impact on female reproductive health [41]. A study of 50 smoking women and 50 nonsmoking women undergoing in vitro fertilization measured the secretion of vascular endothelial growth factor receptor 1 (sVEGFR-1) in follicular fluid. Based on the differences among those two groups, the researchers concluded that smoking is associated with decreased ovarian vascularization and reduced oocyte maturation in smokers [44].
Similarly, air pollution can negatively impact ovarian reserve, as suggested by a study of over 500 Polish women [45] and another study of over 600 women attending Massachusetts General Hospital Fertility Center [46]. Those studies add to a growing body of research linking the negative impact of environmental pollution on female fertility [47].
Medical conditions
Women suffering from various medical conditions might also be at a higher risk of reproductive aging. Such conditions might directly affect the reproductive system and organs or be broader in scope, such as autoimmune diseases.
Endometriosis is a common condition that affects reproductive organ. This chronic inflammatory disease affects roughly 1 in 10 women of reproductive age. Endometriosis owns its name to the endometrium, which creates the uterus lining. In endometriosis, the endometrial cells grow outside of the uterus, leading to pain and infertility and lowering quality of life [48].
Ovarian endometriosis, endometrioma (OMA), is associated with early ovarian aging, including poor ovarian reserve, premature ovarian insufficiency, and early menopause. However, the underlying molecular mechanisms are still poorly understood, and effective treatments are lacking. Available treatments, such as surgical ablation, can “further damage the normal ovarian reservoir, and trigger hyperactivation of primordial follicles, subsequently resulting in the undesired deterioration of ovarian functions” [49].
Hashimoto thyroiditis, also called autoimmune thyroiditis, is an autoimmune disease that is estimated to affect between 5 to 15% of women of childbearing age. Since there are contradictory reports regarding the association between Hashimoto thyroiditis and diminished ovarian reserve, the researchers reviewed, combined, and analyzed the results of multiple studies on the topic. Their conclusions were more nuanced as they observed that “Hashimoto’s thyroiditis negatively affects ovarian reserve in women of reproductive age but not in adolescent and perimenopausal women” [50].
Diabetes also impacts reproductive health. There are differences between type 1 (T1D) and type 2 diabetes (T2D). A few studies that investigated the impact of T2D on ovarian reserve suggest decreased ovarian reserves and accelerated ovarian aging in diabetic females compared to healthy controls [51-53].
Type 1 diabetes is an autoimmune disease. Based on the data from the Familial Autoimmune and Diabetes (FAD) study, the researchers compared women with T1D and their nondiabetic sisters and/or unrelated participants. Those with T1D had a 6-year (17%) reduction in reproductive years due to a combination of a higher probability of being of older age at the time of the first menstruation and a younger age at menopause [54]. However, there is no consensus about the impact of T1D on the age of menopause, as there are also studies that report no differences between women with and without T1D [55].
Some studies report a negative impact of T1D on ovarian reserve. However, there is some inconsistency, as there are also reports suggesting that T1D has a negative impact on ovarian reserve in earlier, but not later, reproductive years. Interestingly, some studies report “that ovarian reserve declines similarly in women with T1DM, regardless of the severity of glucose derangement and the aggressiveness of diabetes treatment,” suggesting that studies on the molecular mechanisms behind the connection between T1D and ovarian aging are indispensable to understand and address these nuanced results [55].
It’s not only about fertility
Reproductive aging comes with multiple health-related challenges that are linked to hormonal changes during menopause. Premature menopause (before age 40 years) or early menopause (between ages 40 and 45 years) is associated with a higher risk of many diseases, including cardiovascular diseases, osteoporosis, depression, dementia, Parkinson’s disease, cognitive impairment, anxiety symptoms, impaired sexual function, and an overall decrease in the quality of life, especially in women who suffer from early-onset and premature menopause [56, 57].
The implications of reproductive aging on fertility and the overall health of women make this an important area of research. However, so far, there are no very successful therapies that extend the female reproductive span.
Literature
[1] Balasch, J., & Gratacós, E. (2011). Delayed childbearing: effects on fertility and the outcome of pregnancy. Fetal Diagnosis and Therapy, 29(4), 263–273.
[2] Soules, M. R., Sherman, S., Parrott, E., Rebar, R., Santoro, N., Utian, W., & Woods, N. (2001). Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertility and Sterility, 76(5), 874–878.
[3] Menken, J., Trussell, J., & Larsen, U. (1986). Age and infertility. Science, 233(4771), 1389–1394.
[4] te Velde, E. R., & Pearson, P. L. (2002). The variability of female reproductive ageing. Human Reproduction Update, 8(2), 141–154.
[5] Hartge, P. (2009). Genetics of reproductive lifespan. Nature Genetics, 41(6), 637–638.
[6] Neff, A. M., Laws, M. J., Warner, G. R., & Flaws, J. A. (2022). The effects of environmental contaminant exposure on reproductive aging and the menopause transition. Current Environmental Health Reports, 9(1), 53–79.
[7] Nehra, D., Le, H. D., Fallon, E. M., Carlson, S. J., Woods, D., White, Y. A., Pan, A. H., Guo, L., Rodig, S. J., Tilly, J. L., Rueda, B. R., & Puder, M. (2012). Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell, 11(6), 1046–1054.
[8] Shadyab, A. H., Macera, C. A., Shaffer, R. A., Jain, S., Gallo, L. C., Gass, M. L. S., Waring, M. E., Stefanick, M. L., & LaCroix, A. Z. (2017). Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women’s Health Initiative. Menopause, 24(1), 35–44.
[9] Sun, F., Sebastiani, P., Schupf, N., Bae, H., Andersen, S. L., McIntosh, A., Abel, H., Elo, I. T., & Perls, T. T. (2015). Extended maternal age at birth of last child and women’s longevity in the Long Life Family Study. Menopause, 22(1), 26–31.
[10] Zhu, Z., Xu, W., & Liu, L. (2022). Ovarian aging: mechanisms and intervention strategies. Medical Review (2021), 2(6), 590–610.
[11] Baker, T. G. (1963). A quantitative and cytological study of germ cells in human ovaries. Proceedings of the Royal Society of London. Series B, Containing Papers of A Biological Character. Royal Society (Great Britain), 158, 417–433.
[12] McGee, E. A., & Hsueh, A. J. (2000). Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews, 21(2), 200–214.
[13] Park, S. U., Walsh, L., & Berkowitz, K. M. (2021). Mechanisms of ovarian aging. Reproduction, 162(2), R19–R33.
[14] Hansen, K. R., Knowlton, N. S., Thyer, A. C., Charleston, J. S., Soules, M. R., & Klein, N. A. (2008). A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Human Reproduction, 23(3), 699–708.
[15] Johnson, J., Canning, J., Kaneko, T., Pru, J. K., & Tilly, J. L. (2004). Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature, 428(6979), 145–150.
[16] Yureneva, S., Averkova, V., Silachev, D., Donnikov, A., Gavisova, A., Serov, V., & Sukhikh, G. (2021). Searching for female reproductive aging and longevity biomarkers. Aging, 13(12), 16873–16894.
[17] Maheshwari, A., Porter, M., Shetty, A., & Bhattacharya, S. (2008). Women’s awareness and perceptions of delay in childbearing. Fertility and Sterility, 90(4), 1036–1042.
[18] Toner, J. P., Grainger, D. A., & Frazier, L. M. (2002). Clinical outcomes among recipients of donated eggs: an analysis of the U.S. national experience, 1996-1998. Fertility and Sterility, 78(5), 1038–1045.
[19] Soares, S. R., Troncoso, C., Bosch, E., Serra, V., Simón, C., Remohí, J., & Pellicer, A. (2005). Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. The Journal of Clinical Endocrinology and Metabolism, 90(7), 4399–4404.
[20] Sampaio, O. G. M., Santos, S. A. A. R., Damasceno, M. de B. M. V., Joventino, L. B., Schneider, A., Masternak, M. M., Campos, A. R., & Cavalcante, M. B. (2024). Impact of repeated ovarian hyperstimulation on the reproductive function. Journal of Reproductive Immunology, 164, 104277.
[21] Khoshnood, B., Bouvier-Colle, M. H., Leridon, H., & Blondel, B. (2008). [Impact of advanced maternal age on fecundity and women’s and children’s health]. Journal de Gynecologie, Obstetrique et Biologie de La Reproduction, 37(8), 733–747.
[22] Kullmer, U., Zygmunt, M., Münstedt, K., & Lang, U. (2000). Pregnancies in Primiparous Women 35 or Older: Still Risk Pregnancies? – Die alte Erstgebärende – eine Risikoschwangerschaft? -. Geburtshilfe Und Frauenheilkunde, 60(11), 569–575.
[23] Barton, J. R., Bergauer, N. K., Jacques, D. I., Coleman, S. K., Stanziano, G. J., & Sibai, B. M. (1997). Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? American Journal of Obstetrics and Gynecology, 176(6), 1236–1240; discussion 1240.
[24] Bobrowski, R. A., & Bottoms, S. F. (1995). Underappreciated risks of the elderly multipara. American Journal of Obstetrics and Gynecology, 172(6), 1764–1767; discussion 1767.
[25] Dildy, G. A., Jackson, G. M., Fowers, G. K., Oshiro, B. T., Varner, M. W., & Clark, S. L. (1996). Very advanced maternal age: pregnancy after age 45. American Journal of Obstetrics and Gynecology, 175(3 Pt 1), 668–674.
[26] Carolan, M. (2003). The graying of the obstetric population: implications for the older mother. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 32(1), 19–27.
[27] Main, D. M., Main, E. K., & Moore, D. H. (2000). The relationship between maternal age and uterine dysfunction: a continuous effect throughout reproductive life. American Journal of Obstetrics and Gynecology, 182(6), 1312–1320.
[28] Huang, L., Sauve, R., Birkett, N., Fergusson, D., & van Walraven, C. (2008). Maternal age and risk of stillbirth: a systematic review. Canadian Medical Association Journal, 178(2), 165–172.
[29] Fretts, R. C., & Usher, R. H. (1997). Causes of fetal death in women of advanced maternal age. Obstetrics and Gynecology, 89(1), 40–45.
[30] Foix-L’Hélias, L., & Blondel, B. (2000). Changes in risk factors of preterm delivery in France between 1981 and 1995. Paediatric and Perinatal Epidemiology, 14(4), 314–323.
[31] Joseph, K. S., Allen, A. C., Dodds, L., Turner, L. A., Scott, H., & Liston, R. (2005). The perinatal effects of delayed childbearing. Obstetrics and Gynecology, 105(6), 1410–1418.
[32] Gracia, C. R., Sammel, M. D., Chittams, J., Hummel, A. C., Shaunik, A., & Barnhart, K. T. (2005). Risk factors for spontaneous abortion in early symptomatic first-trimester pregnancies. Obstetrics and Gynecology, 106(5 Pt 1), 993–999.
[33] Yaegashi, N., Senoo, M., Uehara, S., Suzuki, H., Maeda, T., Fujimori, K., Hirahara, F., & Yajima, A. (1998). Age-specific incidences of chromosome abnormalities at the second trimester amniocentesis for Japanese mothers aged 35 and older: collaborative study of 5484 cases. Journal of Human Genetics, 43(2), 85–90.
[34] Park, I. Y., Kwon, J. Y., Kim, Y. H., Kim, M., & Shin, J. C. (2010). Maternal age-specific rates of fetal chromosomal abnormalities at 16-20 weeks’ gestation in Korean pregnant women >or=35 years of age. Fetal Diagnosis and Therapy, 27(4), 214–221.
[35] Loane, M., Dolk, H., Morris, J. K., & EUROCAT Working Group. (2009). Maternal age-specific risk of non-chromosomal anomalies. BJOG: An International Journal of Obstetrics and Gynaecology, 116(8), 1111–1119.
[36] Eriksson, A. W., & Fellman, J. (2007). Temporal trends in the rates of multiple maternities in England and Wales. Twin Research and Human Genetics, 10(4), 626–632.
[37] Fellman, J., & Eriksson, A. W. (2005). Variations in the maternal age effect on twinning rates: the nordic experience. Twin Research and Human Genetics, 8(5), 515–523.
[38] Dudenhausen, J. W., & Maier, R. F. (2010). Perinatal problems in multiple births. Deutsches Arzteblatt International, 107(38), 663–668.
[39] Ruth, K. S., Day, F. R., Hussain, J., Martínez-Marchal, A., Aiken, C. E., Azad, A., Thompson, D. J., Knoblochova, L., Abe, H., Tarry-Adkins, J. L., Gonzalez, J. M., Fontanillas, P., Claringbould, A., Bakker, O. B., Sulem, P., Walters, R. G., Terao, C., Turon, S., Horikoshi, M., … et al. (2021). Genetic insights into biological mechanisms governing human ovarian ageing. Nature, 596(7872), 393–397.
[40] Ortiz, A. P., Harlow, S. D., Sowers, M., Nan, B., & Romaguera, J. (2006). Age at natural menopause and factors associated with menopause state among Puerto Rican women aged 40-59 years, living in Puerto Rico. Menopause, 13(1), 116–124.
[41] Kaczmarek, M. (2007). The timing of natural menopause in Poland and associated factors. Maturitas, 57(2), 139–153.
[42] Mishra, G. D., Pandeya, N., Dobson, A. J., Chung, H.-F., Anderson, D., Kuh, D., Sandin, S., Giles, G. G., Bruinsma, F., Hayashi, K., Lee, J. S., Mizunuma, H., Cade, J. E., Burley, V., Greenwood, D. C., Goodman, A., Simonsen, M. K., Adami, H.-O., Demakakos, P., & Weiderpass, E. (2017). Early menarche, nulliparity and the risk for premature and early natural menopause. Human Reproduction, 32(3), 679–686.
[43] Langton, C. R., Whitcomb, B. W., Purdue-Smithe, A. C., Sievert, L. L., Hankinson, S. E., Manson, J. E., Rosner, B. A., & Bertone-Johnson, E. R. (2020). Association of parity and breastfeeding with risk of early natural menopause. JAMA Network Open, 3(1), e1919615.
[44] Motejlek, K., Palluch, F., Neulen, J., & Grümmer, R. (2006). Smoking impairs angiogenesis during maturation of human oocytes. Fertility and Sterility, 86(1), 186–191.
[45] Wieczorek, K., Szczęsna, D., Radwan, M., Radwan, P., Polańska, K., Kilanowicz, A., & Jurewicz, J. (2024). Exposure to air pollution and ovarian reserve parameters. Scientific Reports, 14(1), 461.
[46] Gaskins, A. J., Mínguez-Alarcón, L., Fong, K. C., Abdelmessih, S., Coull, B. A., Chavarro, J. E., Schwartz, J., Kloog, I., Souter, I., Hauser, R., & Laden, F. (2019). Exposure to Fine Particulate Matter and Ovarian Reserve Among Women from a Fertility Clinic. Epidemiology, 30(4), 486–491.
[47] Canipari, R., De Santis, L., & Cecconi, S. (2020). Female fertility and environmental pollution. International Journal of Environmental Research and Public Health, 17(23).
[48] Smolarz, B., Szyłło, K., & Romanowicz, H. (2021). Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). International Journal of Molecular Sciences, 22(19).
[49] Tan, Z., Gong, X., Li, Y., Hung, S. W., Huang, J., Wang, C. C., & Chung, J. P. W. (2022). Impacts of endometrioma on ovarian aging from basic science to clinical management. Frontiers in Endocrinology, 13, 1073261.
[50] Li, F., Lu, H., Huang, Y., Wang, X., Zhang, Q., Li, X., Qiang, L., & Yang, Q. (2022). A systematic review and meta-analysis of the association between Hashimoto’s thyroiditis and ovarian reserve. International Immunopharmacology, 108, 108670.
[51] Isik, S., Ozcan, H. N., Ozuguz, U., Tutuncu, Y. A., Berker, D., Alimli, A. G., Akbaba, G., Karademir, M. A., & Guler, S. (2012). Evaluation of ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with type 2 diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism, 97(1), 261–269.
[52] Qin, X., Du, J., He, R., Li, Y., Zhu, Q., Li, Y., Li, H., & Liang, X. (2023). Adverse effects of type 2 diabetes mellitus on ovarian reserve and pregnancy outcomes during the assisted reproductive technology process. Frontiers in Endocrinology, 14, 1274327.
[53] Wang, Y., & Wang, Y. (2022). Accelerated ovarian aging among type 2 diabetes patients and its association with adverse lipid profile. Frontiers in Endocrinology, 13, 780979.
[54] Dorman, J. S., Steenkiste, A. R., Foley, T. P., Strotmeyer, E. S., Burke, J. P., Kuller, L. H., Kwoh, C. K., & Familial Autoimmune and Diabetes (FAD) Study. (2001). Menopause in type 1 diabetic women: is it premature? Diabetes, 50(8), 1857–1862.
[55] Wellons, M. F., Matthews, J. J., & Kim, C. (2017). Ovarian aging in women with diabetes: An overview. Maturitas, 96, 109–113.
[56] Shuster, L. T., Rhodes, D. J., Gostout, B. S., Grossardt, B. R., & Rocca, W. A. (2010). Premature menopause or early menopause: long-term health consequences. Maturitas, 65(2), 161–166.
[57] Orshan, S. A., Furniss, K. K., Forst, C., & Santoro, N. (2001). The lived experience of premature ovarian failure. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 30(2), 202–208.



