The application of artificial intelligence to the study of aging in 2013 led to the development of tools for measuring biological age and predicting mortality, which is defined as the frequency of death in a defined population during a specified interval [1]. Public access to these tools creates the opportunity for self-studies, allowing individuals to gain insights into how their bodies would respond to diet, lifestyle, exercise, and supplementation interventions aimed at changing their biological ages or risks of death.
Artificial intelligence makes measuring age more feasible
In 2013, Steve Horvath developed a highly accurate artificial intelligence-driven method of determining biological age [2]. This long-awaited development ushered in a new era of aging research. For the first time, it enabled researchers in academia and industry to measure the results of their work in terms of changes in biological age.
For example, in 2019, Dr. Greg Fahy and his colleagues carried out an experiment aimed at regenerating the thymus. There were nine participants in the year-long study. In addition to partial regeneration of the participants’ thymi, their average biological age, as measured by DNA methylation patterns, decreased by 2.5 years [3]. Since that time, many AI-based methods for assessing biological age and mortality have been developed.
Public access and self-study
One avenue of biological age testing that is low cost, accessible, and accurate is provided by Levine’s Phenotypic Age Calculator and aging.ai’s 1.0, 2.0, or 3.0 assays. These biological age estimators simply require the user to enter standardized blood test values into an online platform, and it may be possible in the U.S. to cover the cost of such bloodwork through insurance.
What biomarkers offer
Biomarkers provide a starting point for insights into the individual-specific impacts of changes to diet, lifestyle, and exercise habits. For example, a 2015 study investigating post-meal blood glucose found that people have very different glucose responses after eating certain foods [4]. Therefore, anyone attempting to control this would be well served by using a continuous glucose monitor and a food diary.
Glucose is one of the five most relevant biomarkers in predicting biological age and mortality risk; according to Aging.ai, the other four are albumin, alkaline phosphatase, red blood cells, and urea. Therefore, physician-assisted and informed efforts towards lowering high blood sugar can help individuals plan changes in their diet and exercise habits.
Albumin’s decline with age, functional importance, metabolism, and tuning
Of 41 biomarkers included in Aging.ai’s platform, serum albumin has the greatest relative importance. Albumin is a very large protein (69,000 Daltons) that is made in the liver and promptly released into the bloodstream. There, it accounts for roughly 50% of all plasma protein [5]. Albumin’s structural features underscore its remarkable variety of functions, which include pulling water into the circulatory system from tissues (colloid osmotic pressure), transporting different molecules, maintaining pH, neutralizing oxidizing compounds, and providing a reserve of high-quality protein to meet nutritional requirements.
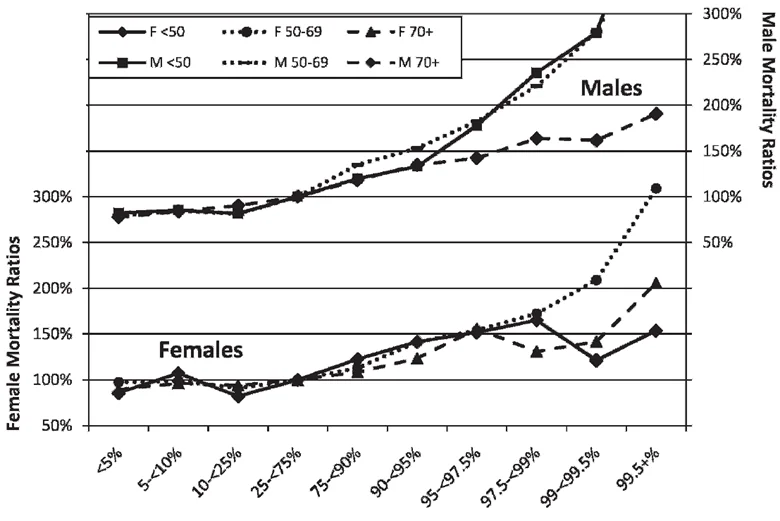
All-cause mortality increases as serum albumin levels decrease with age
Numerous factors can raise or lower serum albumin. Most studies addressing these factors focus on chronic kidney and liver disease, as serum albumin is a key indicator of these conditions. Although there is some controversy on the relationship between aging and albumin levels, a 2015 study by Weaving that looked at 1,079,193 serum albumin results had sufficient data to categorize results by chronological age, sex, and laboratory [6]. Furthermore, a 2010 study by Fulks with 1.7 million subjects found higher serum albumin to be associated with lower all-cause mortality.
These results indicate that serum albumin concentrations are known to decrease with age, and values less than 3.8 grams per deciliter are associated with increased morbidity, mortality, and disability in the elderly [7]. There is a steady increase in serum albumin until puberty in both sexes. During puberty, albumin drops briefly. At adulthood, both sexes peak in their production of albumin but women peak at a lower level that remains lower roughly until menopause. Then, both sexes continue to experience a steady drop for as long as they are alive.

Not everything associated with age predicts mortality. For example, gray hair, wrinkles, and baldness may indicate chronological age but have little or no correlation with premature or delayed mortality [8]. This is not the case with albumin. A study conducted by Fulks of over 1.7 million insurance applicants showed that people with the highest average serum values for their age groups had the lowest risk of mortality and that people with the lowest serum albumin values had the highest risk of mortality [9].
Albumin is a multifunctional protein
Albumin’s functions include blood pressure maintenance, molecular transport, antioxidant defense, pH maintenance, inflammatory control, cell growth and development, and protein storage.
Albumin’s large size and negative charge attracts water, which enables it to maintain colloid osmotic pressure in the circulatory system. Without albumin, fluid would leak out of the blood vessels and into tissues dangerously disrupting normal fluid balance. This condition is known as edema, and if left untreated, it can lead to fibrosis in the affected area and serious circulatory complications.
Albumin transports many kinds of molecules, including fatty acids, metals, free radicals, drugs, and hormones. Albumin carries non-esterified fatty acids, which are the fraction of fatty acids that are not transported with cholesterol inside of LDL and HDL particles. Albumin also transports metals, such as copper and zinc. In the absence of albumin, these metal ions would catalyze the formation of toxic free radicals in serum and other areas. In the same vein, albumin is a scavenger of free radicals; therefore, albumin performs an antioxidant function.
Additionally, while acting as an antioxidant, albumin transports metals to areas where they work as enzyme co-factors. Rather than wreaking havoc on the body, with the aid of albumin, they help normal cell growth and proliferation. Albumin also has binding sites that enable the transport of drugs and hormones such as testosterone. This function is often exploited by bioengineers and pharmaceutical companies as a mechanism for drug delivery.
Another interesting characteristic of albumin is that its shape can be altered by shifts in pH. This characteristic is exploited by the body to provide another mechanism for picking molecules up in one place and dropping them off in others. Finally, albumin functions as a ready source of amino acids for protein synthesis. In fact, fasting can drop normal serum albumin levels by up to 33%, which may affect blood work results.
Albumin metabolism
Serum albumin levels are a function of numerous variables, including albumin production in the liver, hydration, the use of albumin as a protein source, and the presence of other proteins in serum. Decreased production of albumin is a rare cause of low albumin in normal individuals.
Normal serum albumin turnover is approximately 25 days. Albumin turnover reflects a synthesis of albumin in the liver (about 10.5 grams/day), which is offset by kidney (~6%), gut (~10%), and catabolic losses (~84%) [10]. In the absence of liver disease, there is little evidence that the production of albumin is compromised by a decrease in liver function. Rather, lower serum albumin is due to increased losses through the kidney and gut along with other catabolic processes or other factors that affect colloid osmotic pressure.
The fact that albumin is a very large molecule makes it very difficult to excrete through the kidney under normal circumstances. However, approximately 20 milligrams of albumin are lost through urine every day. This has little impact on the 10.5-gram daily total and does not account for the 630 milligrams that are lost daily in the renal system. The other 610 milligrams are catabolized in another area of the kidney by lysosomes [11], the cellular organelles responsible for dissolving proteins. Lysosomal function tends to get worse with age, so it is reasonable for this processing of albumin to decrease with age in the absence of kidney failure.
Many diseases affect the permeability of the gut epithelial cells and the mucosal layer. This can result in losses of albumin greater than the average of ~10%. Such diseases include Crohn’s disease, Kaposi’s sarcoma, and congestive heart failure.
How the other 9.87 grams of albumin are lost each day is not well understood. It is believed that about 60% of this albumin catabolism occurs in the lysosomes of fibroblasts found in skin and muscle.
Another theory is based on the finding that age-related increases in serum proteins are inversely proportional to the normal functional decline seen in aging kidneys [12]. The added protein load leads to marginal increases in colloid osmotic pressure. as colloid osmotic pressure is believed to be the chief regulator of albumin synthesis in the liver [11]. Albumin synthesis could be downregulated to adjust colloid osmotic pressure in diabetics [13].
Factors that alter serum albumin
A mix of positive and negative factors can raise serum albumin. Certain disease states can raise serum albumin. Infectious disease that provokes diarrhea can raise albumin levels via dehydration. In this instance, the relative concentration of albumin in serum goes up, but the absolute concentration is likely unchanged. Failure to drink enough water, eat enough water-rich foods, or excessive sweating for long periods can significantly increase the concentration of serum albumin. However, this would not be considered an advisable approach to increasing serum albumin, considering the adverse and potentially fatal effects of dehydration, such as heat injury, heart attack, kidney and urinary tract issues, hypovolemic shock, gallstones, confusion, fatigue, and dizziness.
Protein intake
Albumin synthesis can be temporarily stimulated by an increased availability of amino acids. This is thought to be a mechanism for both temporarily storing amino acids and protecting them from oxidation [14]. However, the evidence suggests that for individuals with serum albumin levels greater than or equal to 3 grams per deciliter, the relationship between serum albumin and protein intake is negligible [15]. For most people, eating additional protein is not likely to have an appreciable effect on serum albumin levels but may raise blood urea nitrogen considerably, which would tend to increase predicted age based on blood biomarkers.
Strength training
Sarcopenia, the age-related loss of muscle mass, is associated with lower serum albumin [7]. The rate of muscle decline accelerates exponentially from around 8% per decade at 40 years of age to up to 15% per decade after the age of 70. If this loss of muscle mass persists and becomes more severe, it may contribute to the development of frailty, in which the significant loss of muscle mass and strength leads to a high risk of falls along with increased dependency, disability, morbidity, and mortality rate [16].
Strength training potentially offers multiple age-related benefits, including increased muscle mass, strength, lower body fat, improved bone density, improved coordination, and mood [17]. A meta-analysis that looked at the potential of strength training to address the effects of sarcopenia showed that strength training effectively reduced body fat mass and improved muscle strength and performance in healthy older people with sarcopenia. However, increases in lean muscle mass were not observed in this meta-analysis [18,19].
Studies looking at the effects of strength training on serum albumin have yielded mixed results. For example, strength training In patients who had chronic kidney disease but were not dependent on dialysis has been shown to increase serum albumin [20]. While strength training in young men has been shown not to affect serum albumin [21], individuals with moderately low albumin have been shown to have weaker muscles and relatively reduced strength [22].
Supplements recommended in the literature for sarcopenia
Creatine monohydrate increases available phosphocreatine, which is necessary for high-intensity strength and power exercises. Studies in older people have provided some evidence of the positive effects of creatine. In a study of 30 men older than 70, creatine supplementation increased lean tissue mass, increased leg strength, power, and endurance [23,24].
Beta-hydroxy-beta-methylbutyrate (HMB), a breakdown product of the amino acid leucine, has been shown in several studies to counteract sarcopenia by stimulating growth signaling pathways and inhibiting muscle breakdown. Leucine does this by directly activating the mammalian target of rapamycin (mTOR) pathway and inhibiting the organelles that break down protein [16]. Evidence suggests that sarcopenia may be countered through any combination of strength training, creatine, and HMB to maintain optimal serum albumin levels, given the association between sarcopenia and muscle mass. However, the association with leucine and mTOR activation could cause more harm than good if the overarching objective is to slow aging, as mTOR inhibition is associated with increased longevity in many species [25].
Some dairy products may improve gut barrier function to reduce albumin losses
An estimated 60-70 million Americans, or about 1 in 5 Americans, are diagnosed with some form of gastrointestinal disease each year [26]. Suboptimal barrier function is a common feature in these diseases, and it may increase losses of albumin through the gut barrier via two mechanisms: inflammation, which decreases albumin synthesis, and enhanced permeability or leakiness of the gut. Therefore, improving barrier function could improve albumin synthesis and prevent additional losses through the gut.
Poor barrier function is often associated with poor eating habits, including inadequate fiber and other nutrients. Fiber is fermented by gut bacteria to produce butyrate, a prime energy substrate for intestinal cells (enterocytes). When butyrate production is insufficient, barrier function can be compromised. Conversely, when butyrate production is enhanced through increased fiber consumption, gut barrier health may be improved.
Another method of improving gut barrier health is through the consumption of yogurt. Yogurt contains numerous probiotics that contribute to improved barrier function and even promote weight loss [27]. If the yogurt contains vitamin D, as most varieties do, this may further improve gut function. About 24% of people in the United States are deficient in Vitamin D [28].
Vitamin D may improve gut barrier function through multiple mechanisms, including better regulation of tight junction proteins that hold gut cells together and the prevention of programmed cell death (apoptosis) [29]. Yogurt also contains a species of lactobacillus (milk bacteria) that increases the production of immunoglobulin A, an antibody that protects the mucosal layer of the intestine from microbial invasion. Finally, tryptophan, an amino acid present in large quantities in dairy products, has been shown to increase albumin synthesis in rabbit liver by up to 178% [30].
Dr. Mike Lustgarten, advocate and practitioner of biomarker optimization, recently stated that adding full-fat yogurt to his diet caused his serum albumin to increase too much. When he stopped eating cheese and switched to low-fat yogurt, his albumin dropped. Dr. Lustgarten also reports a strong positive correlation between beta carotene and serum albumin [31].
Diabetes
Insulin regulates albumin gene transcription by inhibiting the expression of the FoxO1 gene. FoxO1 acts as a repressor of albumin expression [32]. In both types of diabetes, insulin production becomes compromised, but in Type 2 diabetes, insulin production may initially increase to overcome insulin resistance. During this phase, serum albumin may increase, since insulin production is higher than normal. As pancreatic beta cells begin to burn out, insulin production would then be expected to become progressively lower. This logically leads to an increase in FoxO1 gene expression, thus leading to inhibition of albumin expression and lower serum albumin.
Therefore, people with poorly controlled diabetes may have reduced synthesis of albumin as well as elevated levels of glucose. Improving glucose control may therefore also lead to a rise in serum albumin. Higher albumin is associated with a younger biological age, but it may be possible to increase albumin synthesis by increasing insulin secretion. However, just because synthesis is decreased does not necessarily mean that serum albumin will decrease, as losses of albumin may be protected against in other ways.
Damaged kidneys
Damage to small clusters of blood vessels in the kidney may result in excessive loss of albumin through the urine (proteinuria). Uncontrolled high blood pressure, excessive use of painkillers, excessive phosphorus intake, dehydration, lack of sleep, overeating, smoking, high-sugar diets, and excessive alcohol can damage the kidneys.
Liver damage
Low albumin is a feature of chronic and advanced liver scarring (hepatic cirrhosis). The liver is the sole producer of albumin, and liver damage can have an impact on albumin synthesis. Alcohol, hepatitis, drugs, acute injury, and many other factors can lead to cirrhosis.
Heart failure
Heart failure can be caused by damage due to a heart attack or through exposure to toxins such as alcohol. Poor liver function can result in higher circulating levels of various toxins that induce heart failure. Low albumin is common in individuals with cardiac failure because the inability of the heart to pump blood causes fluid to back up, disrupting albumin distribution throughout the body.
Inflammation
Systemic inflammation increases the loss of serum albumin by increasing the leakiness of capillaries. This increased capillary leakage is due to cytokines such as TNF-alpha and IL-6, along with chemokines, prostaglandins, and endotoxins from Gram-negative bacteria [11,33].
This leads to an expansion of the space between cells in normal tissue (edema) [34,35]. Systemic inflammation can be associated with many different conditions, including the age-related accumulation of senescent cells. The rate of albumin synthesis is also decreased in critical illness, and this is thought to be a result of an increase in gene transcription of proteins such as C-reactive protein and a decreased rate of transcription of albumin mRNA [33].
Literature
[1] J. Hernandez and P. Kim, Epidemiology Morbidity and Mortality. Treasure Island (Fl): StatPearls Publishing, 2022
[2] S. Horvath, “DNA methylation age of human tissues and cell types,” Genome Biol., vol. 14, no. 10, p. 3156, 2013
[3] G. M. Fahy et al., “Reversal of epigenetic aging and immunosenescent trends in humans,” Aging Cell, vol. 18, no. 6, p. e13028, Dec. 2019
[4] D. Zeevi et al., “Personalized Nutrition by Prediction of Glycemic Responses,” Cell, vol. 163, no. 5, pp. 1079–1094, 2015
[5] A. Farrugia, “Albumin Usage in Clinical Medicine: Tradition or Therapeutic?” Transfus. Med. Rev., vol. 24, no. 1, pp. 53–63, 2010
[6] G. Weaving, G. F. Batstone, and R. G. Jones, “Age and sex variation in serum albumin concentration: an observational study,” Ann. Clin. Biochem., vol. 53, no. 1, pp. 106–111, Jun. 2015.
[7] R. N. Baumgartner, K. M. Koehler, L. Romero, and P. J. Garry, “Serum albumin is associated with skeletal muscle in elderly men and women,” Am. J. Clin. Nutr., vol. 64, no. 4, pp. 552–558, Oct. 1996
[8] P. Schnohr, J. Nyboe, P. Lange, and G. Jensen, “Longevity and Gray Hair, Baldness, Facial Wrinkles, and Arcus Senilis in 13,000 Men and Women: The Copenhagen City Heart Study,” Journals Gerontol. Ser. A, vol. 53A, no. 5, pp. M347–M350, Sep. 1998
[9] M. Fulks, R. Stout, and V. Dolan, “Albumin and all-cause mortality risk in insurance applicants,” J. Insur. Med., vol. 42, no. 1, pp. 11–17, 2010
[10] J. Lang et al., “Association of serum albumin levels with kidney function decline and incidence of chronic kidney disease in elders,” Nephrol. Dial. Transplant, vol. 33, no. 6, pp. 986–992, Jun. 2018
[11] D. G. Levitt and M. D. Levitt, “Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements,” Int. J. Gen. Med., vol. 9, pp. 229–255, Jul. 2016
[12] L. Lind, J. Sundström, A. Larsson, E. Lampa, J. Ärnlöv, and E. Ingelsson, “Longitudinal effects of aging on plasma proteins levels in older adults – associations with kidney function and hemoglobin levels,” PLoS One, vol. 14, no. 2, pp. e0212060–e0212060, Feb. 2019
[13] D. E. McMillan, “Increased levels of acute-phase serum proteins in diabetes,” Metabolism, vol. 38, no. 11, pp. 1042–1046, 1989
[14] P. De Feo, F. F. Horber, and M. W. Haymond, “Meal stimulation of albumin synthesis: a significant contributor to whole body protein synthesis in humans,” Am. J. Physiol. Metab., vol. 263, no. 4, pp. E794–E799, Oct. 1992
[15] S. Sarwar and R. A. Sherman, “How Well Does Serum Albumin Correlate with Dietary Protein Intake in Dialysis Patients?,” Kidney Int. reports, vol. 2, no. 1, pp. 90–93, Sep. 2016
[16] J. Oktaviana, J. Zanker, S. Vogrin, and G. Duque, “The Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review,” J. Nutr. Health Aging, vol. 23, no. 2, pp. 145–150, 2019.
[17] R. Seguin and M. E. Nelson, “The benefits of strength training for older adults,” Am. J. Prev. Med., vol. 25, no. 3, Supplement 2, pp. 141–149, 2003
[18] J. Silva-Fhon, V. Rojas-Huayta, J. Aparco-Balboa, and R. Céspedes-Panduro, B Partezani-Rodrigues, “Sarcopenia and blood albumin: A systematic review with meta-analysis,” Biomedica, vol. 41, no. 3, pp. 590–603, 2021
[19] N. Chen, X. He, Y. Feng, B. E. Ainsworth, and Y. Liu, “Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials,” Eur. Rev. Aging Phys. Act., vol. 18, no. 1, p. 23, 2021
[20] I. Moinuddin and D. J. Leehey, “A Comparison of Aerobic Exercise and Resistance Training in Patients With and Without Chronic Kidney Disease,” Adv. Chronic Kidney Dis., vol. 15, no. 1, pp. 83–96, 2008
[21] M. Sheffield-Moore et al., “Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise,” Am. J. Physiol. Metab., vol. 288, no. 5, pp. E922–E929, May 2005
[22] B. W. M. Schalk, D. J. H. Deeg, B. W. J. H. Penninx, L. M. Bouter, and M. Visser, “Serum Albumin and Muscle Strength: A Longitudinal Study in Older Men and Women,” J. Am. Geriatr. Soc., vol. 53, no. 8, pp. 1331–1338, Aug. 2005
[23] R. Cannataro et al., “Sarcopenia: Etiology, Nutritional Approaches, and miRNAs,” International Journal of Molecular Sciences, vol. 22, no. 18. 2021
[24] J. E. Morley et al., “Nutritional Recommendations for the Management of Sarcopenia,” J. Am. Med. Dir. Assoc., vol. 11, no. 6, pp. 391–396, 2010
[25] N. Mota-Martorell, M. Jové, and R. Pamplona, “mTOR Complex 1 Content and Regulation Is Adapted to Animal Longevity,” International Journal of Molecular Sciences, vol. 23, no. 15. 2022
[26] A. F. Peery et al., “Burden of Gastrointestinal Disease in the United States: 2012 Update,” Gastroenterology, vol. 143, no. 5, pp. 1179-1187.e3, 2012
[27] R. Pei, D. A. Martin, D. M. DiMarco, and B. W. Bolling, “Evidence for the effects of yogurt on gut health and obesity,” Crit. Rev. Food Sci. Nutr., vol. 57, no. 8, pp. 1569–1583, May 2017
[28] K. Amrein et al., “Vitamin D deficiency 2.0: an update on the current status worldwide,” Eur. J. Clin. Nutr., vol. 74, no. 11, pp. 1498–1513, 2020
[29] C.-Y. Yeung et al., “Effects of Vitamin D-Deficient Diet on Intestinal Epithelial Integrity and Zonulin Expression in a C57BL/6 Mouse Model,” Front. Med., vol. 8, p. 649818, Aug. 2021
[30] M. A. Rothschild, M. Oratz, J. Mongelli, L. Fishman, and S. S. Schreiber, “Amino Acid Regulation of Albumin Synthesis,” J. Nutr., vol. 98, no. 4, pp. 395–403, Aug. 1969
[31] M. Lustgarten, “Albumin: What’s Optimal For Youth And Health? (2022 Update),” 2022
[32] Q. Chen, M. Lu, B. R. Monks, and M. J. Birnbaum, “Insulin Is Required to Maintain Albumin Expression by Inhibiting Forkhead Box O1 Protein,” J. Biol. Chem., vol. 291, no. 5, pp. 2371–2378, Jan. 2016
[33] V. Gouden, V. Rishik, and I. Jialal, “Hypoalbuminemia,” Stat Pearls , 2022
[34] V. Arroyo, R. García-Martinez, and X. Salvatella, “Human serum albumin, systemic inflammation, and cirrhosis,” J. Hepatol., vol. 61, no. 2, pp. 396–407, 2014
[35] P. B. Soeters, R. R. Wolfe, and A. Shenkin, “Hypoalbuminemia: Pathogenesis and Clinical Significance,” JPEN. J. Parenter. Enteral Nutr., vol. 43, no. 2, pp. 181–193, Feb. 2019



