In downtown San Francisco, an entire tower is becoming a hub for longevity, AI, crypto, and robotics. It just hosted its first longevity conference.
How does a group conquer a big city?
The longevity community unites tens of thousands of researchers, founders, and enthusiasts, but this is mostly done virtually. Things began to change after Zuzalu, a first-of-its-kind “pop-up city” (rather village), which existed in Montenegro for two months in 2023. Funded largely by the multi-millionaire founder of Ethereum, Vitalik Buterin, it was a gathering of several hundred eager, mostly young people with somewhat overlapping interests, including crypto, AI, and longevity.
Zuzalu was inspired in part by the ideas of Balaji Srinivasan, former CTO of Coinbase. Balaji, as he is referred to in these circles, coined the term “network state”: a new type of community designed to bring like-minded people together, both virtually and physically. The term refers to a sprawling concept that outlines several principles, such as having a unifying idea, but it hardly prescribes any particular realization. Theoretically, network states can have either a lot of, some, or no physical presence.
After Zuzalu, many alumni immediately set off to improve the formula. Some recreated it rather faithfully with minor tweaks. Others eyed “taking over” Rhode Island using political action. Vitalia, a two-month-long longevity-oriented event on the tropical island of Roatan, was a notable step forward for two reasons. First, it was based on (and in) the free economic zone of Prospera, which has loose regulations that proved to be a great fit for biotech startups, enabling them to start selling their therapies after a successful Phase 1 trial. Second, a small permanent community was established after the several-hundred-strong “pop-up city” phase ended.
While places like Prospera, which creatively juggle regulations and are somewhat of a state within a state, are attractive, they can only be found (for now) in countries far away from the centers of science and innovation. Getting closer to those centers means hitting barriers like a high cost of living.
Then, two things happened in rapid succession. From March to May, the Vitalism movement held Vitalist Bay, a pop-up village in Berkely, California, featuring a series of weekly longevity-related conferences. Just as Vitalist Bay ended, and its residents dispersed, another project nearby kicked off, and it was even weirder – in a good way.
It takes a vertical village
In March, three young German entrepreneurs, Jakob Drzazga, Christian Nagel, and Christian Peters, bought a 16-story tower in the Mid-Market area of San Francisco’s downtown, one of the city’s infamous hotspots of homelessness and the ongoing fentanyl crisis. Real estate prices in the neighborhood have seen giant swings in recent years: the three buyers shelled out 11 million dollars, which is roughly double the building’s previous selling price from 2023 but much lower than the $62 million tag it carried less than ten years ago. Still, Jakob and the two Christians had to borrow heavily, basically betting their future on the project.
Their idea is to transform the tower into the ultimate co-working and co-ideation space for people engaged in several cutting-edge areas, such as crypto, AI, robotics, and longevity. As of now, the tower technically does not offer co-living, but some kind of an arrangement will probably be reached.
Each floor has a purpose. There’s a robotics lab, a biochemistry lab, a gym, a relaxation space, and so on. 11th is the longevity floor, run by no other than Laurence Ion, longevity investor and entrepreneur, co-creator of VitaDAO, and an organizer of both Zuzalu and Vitalia.
Laurence’s overarching goal is to build a real, physical longevity city. There’s a name – Viva City – but not much more. However, you can already get early membership. Laurence also offers $1 million for facilitating a fateful contact with the leadership of a state that chooses to lend a piece of its territory for this project.
Meanwhile, Laurence used the tower for a slightly less ambitious project: a ‘vertical pop-up village’ called Viva Frontier Tower, centered around longevity, AI, and crypto, and touted on its website as “the healthiest, most productive 6 weeks of your life.” In a tried and tested fashion, every section is built around a conference (it seems like all conferences are now called “summits”), which can hopefully attract top-tier speakers and enough paying audience to not bankrupt the organizers.
I was invited and flew in from Seattle the day before. Judging by the map, the location was amazing: in the heart of the downtown, just a couple of blocks away from a BART station. I love trains, but in this case, considering the late time and what I knew about the destination, I grudgingly took an Uber. When I walked out of the hotel next morning, I realized I’d made a wise decision.
Mid-Market might be slowly getting better, but it’s still very much unwell, full of gut-wrenching scenes of miserable, broken, ill people let down by the society they live in. However, the crisis made the real estate cheap, which made buying the tower possible, which might, with time, help steer the area towards better fortunes.
Laurence confirmed to me that this is the idea: “The neighbors can be… interesting. It’s not the most pleasant first impression for some people, but I don’t think it’s dangerous. They are mostly harmless people on drugs. We are revitalizing the area. After COVID, many businesses closed, and the area became empty. We are reclaiming the empty spaces for the community.”
The summit on the 11th floor
The conference started with a rooftop rave and continued with a quirky mix of talks ranging from hard science to wellness to politics to topics that would have been deemed completely outlandish until very recently. Laurence kicked off the action by presenting the conference and talking about VitaDAO, probably the most successful decentralized science project in the longevity space.
On the science-heavy side, we had people like Amit Sharma, a senior researcher at the Lifespan Research Institute (formed by the merger of SENS Research Foundation and Lifespan.io). Amit presented his lab’s cool research into cellular senescence, which we have covered extensively. The list of big names in longevity who had chosen to appear, lending a lot of credibility to the new conference, also included Aubrey de Grey, Greg Fahy, Marco Quarta, Irina Conboy, Matthew O’Connor, and others.
Aubrey, known for his ability to always read the room, chose to present without slides, forgoing the scientific stuff, such as his foundation’s Robust Mouse Rejuvenation project. Instead, he gave a fast-paced, passionate talk centered on how to promote the longevity cause and aptly named “How can we make aging the new COVID?” In particular, he weighed in on the raging debate on whether the longevity field should be wary of overpromising and underdelivering when trying to recruit public support.
According to Aubrey, developing working interventions first and presenting them to the public as the basis for asking for support and money “is not the only way forward.” Cancer research, he said, boomed after the “War on Cancer” was declared more than half a century ago, even though oncologists had little idea of how to actually tackle the disease. They wildly overpromised and underdelivered, yet this seemed to only reinforce society’s resolve to find the cure. For decades, immense funds poured in, and today, we have finally made some real progress. Why should the war against aging be any different? If anything, we now have more understanding of aging than we had of cancer 50 years ago and more working interventions, at least in preclinical models.
A notable appearance was made by Ryan Field from Kernel, Bryan Johnson’s company that has been quietly working for several years on measuring the brain. Ryan presented not just his company’s vision, but the finished product: a neat-looking fabric-clad grey-and-black helmet that Kernel has started to market to clinics and research institutions. The helmet “generates detailed maps of activity in the brain that provide a deep and scalable understanding of disease state and treatment response.” In particular, Ryan touted functional measurements of mild clinical impairment (MCI) and depression. The tech uses light to measure oxygen in brain tissue, Ryan said.

Ryan Field from Kernel holding the helmet, which is part of the company’s Flow2 platform
Impressive as the helmet was, it couldn’t match the audacity of not one but two companies present at the conference that are actually working on mind uploading: creating a computer-based copy of the human brain that is faithful enough to serve as your ‘electronic double.’ Mind uploading has been thought to belong entirely to science fiction for the foreseeable future, but, apparently, there are actual companies with actual funding who think that this mind-blowing goal is already within our reach.
Both Jessica Radley from Nectome and Michael Andregg from Eon Systems mentioned one of the most impressive scientific feats of the last decade: completing the connectome (full map of the neuronal connections) of a Drosophila fly’s brain, now available online. Both companies are building on it to create the connectome of a human brain, while Nectome also looks towards preserving the genome, transcriptome, epigenome, and, basically, “every biological macromolecule” in the brain to faithfully reproduce a human’s personality. Today, the cost of the compute is still prohibitively high, but Jessica and Michael hope that Moore’s law will continue to hold true.
Michael made a suspiciously rosy prediction: the connectome of a mouse brain will be completed as soon as the next year, and a human one will be done over the next few years. However, there’s a major catch: with Eon System’s tech, it’s a one-way ticket to virtual existence. To map the connections, the brain must be artificially enlarged and sliced; this is how the fly’s brain was analyzed, using microscopes. There’s no way back to the physical world from there: in the best-case scenario, “you” will end up in a virtual reality built by Eon. Michael promised that it will be fun.

Jessica Radley from Nectome presenting. Behold a fly’s brain!
Like several other recent conferences, this one veered quite a bit towards politics and public policy. If you think mind uploading is a long shot, so are the political ambitions of Zoltan Istvan, a transhumanistic author and politician who gained some notoriety running for presidency in 2016 for the Transhumanist Party. In 2018, he ran for Governor of California for the Libertarian party; in 2020, he challenged Trump in the Republican Party’s presidential primaries; and, finally, earlier this year, Zoltan announced another run for California governorship, this time as a Democrat. So, he has been a member of four parties in total in seven years. Zoltan is also quite outspoken about extreme longevity, having starred in the documentary Immortality or Bust.
From the conference’s pulpit, Zoltan outlined his program, which actually made a lot of sense to me. Zoltan is one of the handful of political figures who understand the enormity of the impact that AI is going to have on our society in a matter of years and the need to protect people from the resulting massive job loss, building an economy of AI-based abundance. Education, healthcare, and the social safety net will all have to be heavily modified for this new era, which will also rush in new possibilities for fighting aging. Unfortunately, nation-wide, Zoltan’s warnings and ideas will probably fall on deaf ears, and mainstream politicians will continue to play catch-up with the reality of the AI revolution.
One tower fits all
During one of the breaks, I joined a tour of the tower led by Laurence. The building’s age is showing, but it’s holding fairly well. WeWork used to be one of the residents, and several floors still have an entire co-working infrastructure in place: offices, chairs, tables, beanbags, conference rooms. This already promises a nice return on your $190 monthly fee if you live in the area and are looking for a coworking arrangement. The membership also gives you access to the gym. Here’s where the tower’s uniqueness kicks in: pay a bit extra, and you’ll have access to the wet lab! If this is still too steep, there are scholarships.
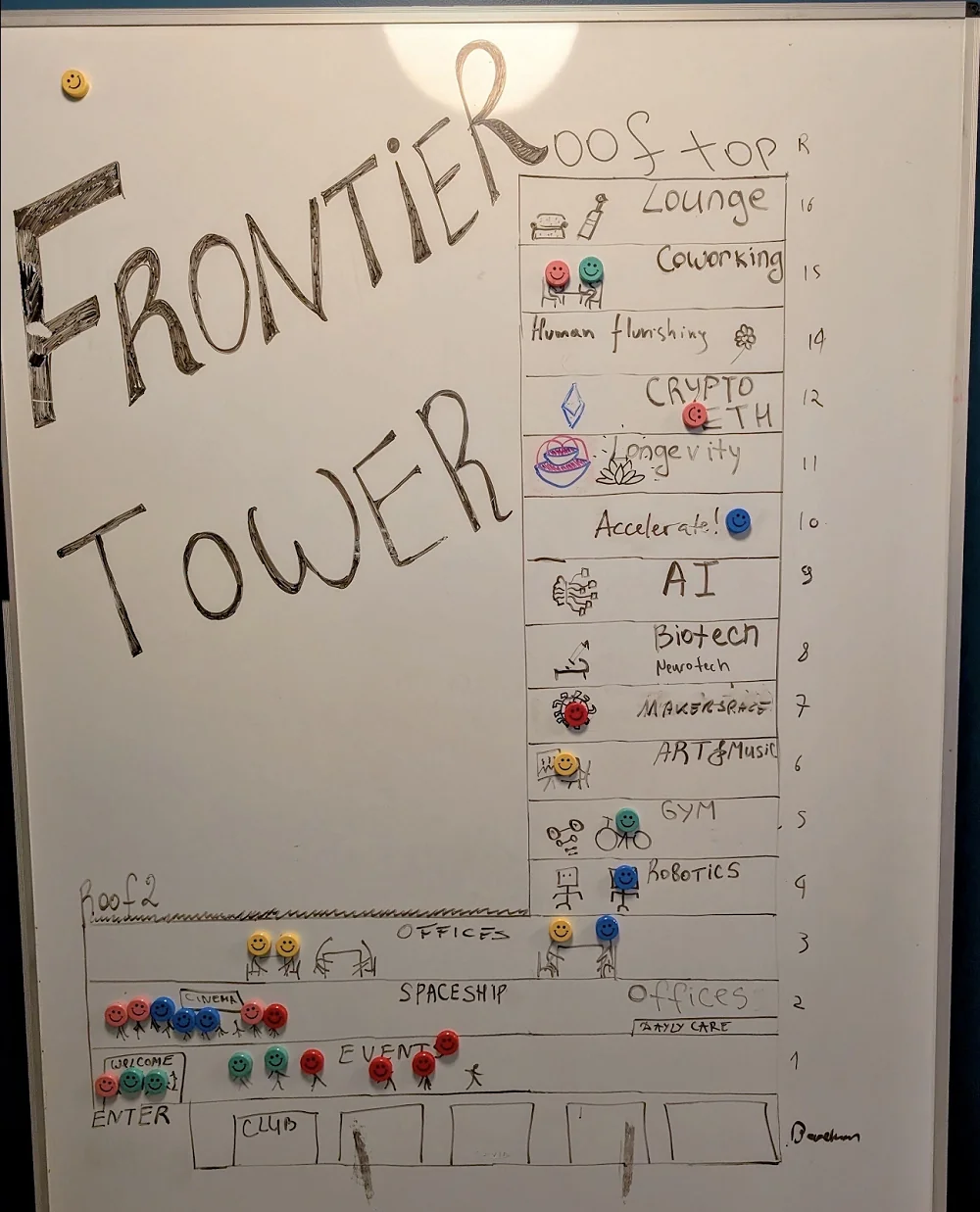
The tower’s plan. Fancy visiting the Spaceship?
“It’s the first permanent space for the longevity biotech community where people can co-work, hold events, exercise, and have healthy food together,” Laurence said. “We’re progressively adding more facilities, like a biohacking clinic, a nootropics bar, biological age testing, blood storage, IV therapy, a DEXA scanner, and hyperbaric oxygen therapy. We’ve pivoted slightly to focus on all frontier tech communities, not just longevity. This brings in much more energy. There are many people in crypto and AI who want to live longer; they might not come specifically for a longevity floor, but they come here for the broader community and end up engaging with and helping the longevity field.”
Like probably every second building in San Francisco, the tower hosts a startup incubator program. How does it work? “We hold sessions with mentors who help companies prepare for a demo day where they can present to investors and hopefully raise money,” Laurence said. “The vast majority of the companies currently in the program are longevity biotech companies.”
However, the tower is also half-empty, scrappy, with signs of ongoing construction. It looks like a work in progress and like it would take a serious effort to fulfill its undeniable promise. Laurence, however, is optimistic: “It’s going well, though it’s not in the bag yet. There’s a lot of buzz, and people in the city and the tower love it, so it feels like a success. The tower is buzzing, at least during the pop-up city events. I hope this feeling will continue as people get used to the space and feel like it’s a true community home, not just a coworking space you visit once a week. People are really connecting here. We’re already talking about expansion, perhaps to a hotel nearby.”

The longevity floor sports a quirky semi-industrial design.
Ich bin ein Berliner
The founders’ origin is not the only reason the startup behind the Frontier Tower is called BerlinHouse. They apparently drew inspiration from the reunification of the German capital in the late 20th century.
“After the fall of the Berlin Wall, tens of thousands of people left East Berlin, leaving hundreds of buildings unoccupied,” the tower’s website says. “This vacancy gave rise to vibrant communities that created clubs, art spaces, collectives, and hacker houses which make Berlin so special. Today, a similar opportunity is emerging in downtowns worldwide, where empty office spaces are becoming the new frontier. Now is our chance to reclaim these spaces, redefine urban living, and foster deeper connections in our cities.”
They have a point. Opportunities are indeed emerging. Despite the attempts by many big companies to bring their workers back to office, the centrifugal force of remote work is probably already unstoppable, and the advance of AI is poised to accelerate this process even more. As towers in the world’s cities get emptied, they might become the pillars of future network states.
Jakob Drzazga, CEO of BerlinHouse, said that the project was “an opportunity to fundamentally rethink how we use buildings,” and that much of the inspiration came directly from earlier pop-up city experiments like Zuzalu. It’s not just about space usage.
Co-habitation Zuzalu-style allows people to enjoy the best of both worlds: collaboration at almost the speed of online tools like Zoom with all the benefits of live, offline communication. I’ve experienced first-hand how talking to other people and even watching them work can snap people out of procrastination or writer’s block.
Even though his hands are full with the Frontier tower, Laurence continues to pursue the much more ambitious vision of Viva City. “Viva City is a bigger project, and we are growing as a community while talking to governments about potential locations,” he said. “To grow the community, we are building a ‘one-stop shop’ product for longevity and seeding these towers in major cities as hubs for the Viva City community and our extended family.”
Apparently, you don’t have to choose between a faraway destination and a bustling downtown. You need them both. “You still need those special jurisdictions for regulatory freedom,” Laurence admitted. “But it’s also important to have hubs in every major city where people can get a taste of the community. They can come into a local space, learn about the vision, and realize that if they truly want to innovate, they need to go to the new frontier – that crazy island in the middle of nowhere.”
We would like to ask you a small favor. We are a non-profit foundation, and unlike some other organizations, we have no shareholders and no products to sell you. All our news and educational content is free for everyone to read, but it does mean that we rely on the help of people like you. Every contribution, no matter if it’s big or small, supports independent journalism and sustains our future.